Macular oedema
Peer reviewed by Dr Colin Tidy, MRCGPLast updated by Dr Hayley Willacy, FRCGP Last updated 16 Jan 2024
Meets Patient’s editorial guidelines
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find the Visual problems article more useful, or one of our other health articles.
In this article:
Continue reading below
What is macular oedema?
Macular oedema consists of intra-retinal or subretinal fluid accumulation in the macular region. It occurs during the course of numerous retinal disorders and can cause severe impairment of central vision.
Major causes of macular oedema include diabetes, branch and central retinal vein occlusion, choroidal neovascularisation, posterior uveitis, postoperative inflammation and central serous chorioretinopathy.1
The pathophysiological process is a breakdown of the blood-retinal barrier (BRB), which normally prevents water movement in the retina, thus allowing fluid to accumulate in the retinal tissue. Inflammatory processes and an increase in vascular permeability play a central role.
Different mechanisms, complicated by ischaemic conditions, interact in a complex manner. Key factors are angiotensin II, prostaglandins and the vascular endothelial growth factor (VEGF).2
The macula is the part of the retina responsible for sharp, central vision due to its high density of cone photoreceptors. It is situated at the back of the retina (the posterior pole), lying about 3 mm lateral to the optic disc. It has a central depression known as the fovea centralis.
The fovea contains tightly packed cone photoreceptors in the fovea with no overlying blood vessels. This is the area of the retina where visual acuity is ultimately determined, where reading takes place and where form, shape and colour are most accurately detected. Macular fluid accumulation alters cell function in the retina as well as provoking an inflammatory reparative response.3
Diagram detailing the macula
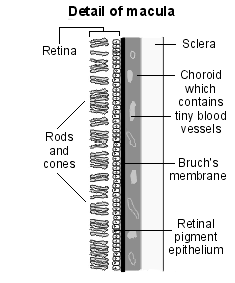
The severity of macular oedema is determined by:4
The extent of the oedema.
The distribution in the macular area (ie focal versus diffuse).
Central foveal involvement.
Evidence of alteration of the BRB and intraretinal cysts.
Signs of ischaemia.
Presence or absence of vitreous traction.
Increase in retinal thickness and cysts in the retina.
Chronicity (ie time elapsed since initial diagnosis).
Macular oedema is usually described as two or three subtypes, depending on the underlying pathophysiology and on the structural changes which result.
Cystoid macular oedema (CMO) with fluid accumulation in cyst-like spaces in the outer plexiform layer of the macula. This condition is an endpoint for many intraocular conditions.
Diabetic macular oedema (DMO) caused by leaking macular capillaries. DMO may lead to CMO.
Macular oedema associated with age-related macular degeneration (AMD), which may also lead to CMO.
Cystoid macular oedema (CMO)5 6
CMO is characterised by intraretinal oedema contained in honeycomb-like (cystoid) spaces filled with clear fluid. The underlying cause is thought to be disruption to the BRB. Retinal cells are displaced by the cysts, so the fluid affects both cell function and cell architecture.
Depending on the aetiology, CMO is usually self-limiting and spontaneously resolves within 3-4 months. Resolution may be helped via medical or surgical options. If the oedema is chronic (more than 6-9 months) permanent damage to photoreceptors with retinal fibrosis can occur.
CMO is a common 'endpoint' pathological response to a variety of insults to the macular area:
Ocular inflammatory diseases - eg, uveitis, pars planitis, Behçet's syndrome, toxoplasmosis and HIV-related cytomegalovirus (CMV) uveitis. It seems likely that inflammatory mediators, particularly CD4+ T cells, are an integral part of the pathological process, causing focal areas of BRB breakdown.
Postoperative CMO following cataract surgery.7 Macular oedema occurs commonly after cataract surgery, even in the absence of complications and risk factors.8 It is thought to occur as a result of release of inflammatory mediators within the eye. If prolonged, permanent damage can occur. However, significant decrease in visual acuity is seen only in about 1%. If cataract extraction is complicated there is a significantly higher incidence. Cataract surgery in patients with diabetes may result in dramatic acceleration of pre-existing diabetic macular oedema (DMO).
Central and branch retinal vein occlusions. In these conditions a rise in intravenous and capillary pressure leads to stagnation of blood, hypoxia of the affected structures and damage to the capillary endothelial cells, with extravasation of plasma constituents.
Other causes of CMO include retinal vascular disease (eg, idiopathic retinal telangiectasia), retinal dystrophies (eg, retinitis pigmentosa) and drug-induced changes (as can occur with topical adrenaline (epinephrine) 2%, particularly in patients without a lens).
CMO can also occur following injury to the eye and in association with choroidal tumours.
Diabetic macular oedema (DMO)
DMO occurs in the context of diabetic retinopathy, both proliferative and non-proliferative types. If it occurs in a critical part of the macula or reaches a particular size, it is referred to as clinically significant macular oedema (CSMO). DMO is the major cause of vision loss in diabetes.
Macular degeneration leading to macular oedema9
Patients with certain forms of AMD - exudative ('wet') AMD - are prone to macular oedema. AMD gives rise to formation of choroidal neovascular membranes which leak fluid and blood under the retina, so resulting in macular oedema. See the separate Age-related Macular Degeneration article for more detail . The presence of CMO and foveal thickening is associated with worse visual acuity.
What happens in macular oedema? (Pathophysiology)2
Cystoid macular oedema (CMO)
The vitreous, retina, retinal pigment epithelium (RPE) and choroid receive their circulation through the retinal and choroidal vasculature. This relies upon an intrinsic balance amongst the osmotic force, hydrostatic force, capillary permeability and tissue compliance in the vasculature..
Once imbalance occurs, an accumulation of fluid is seen in cystoid spaces within the inner layers of the retina. Vitreomacular traction is a common underlying factor which contributes to the release of inflammatory factors such as VEGF and platelet-derived growth factor (PDGF). This results in BRB breakdown, leakage and oedema.
Diabetic macular oedema (DMO)10
Diabetic retinopathy is a neurovascular disease of the retina, and retinal neuronal abnormalities precede the retinal microvascular injury. Increased vasopermeability with breakdown of the BRB is due to many factors, including upregulation of the expression of VEGF and altered vitreoretinal interface with persistent vitreomacular traction (VMT).
Blood vessel damage plays a significant role. Long-term hyperglycaemia leads to vascular basement membrane thickening, non-enzymatic glycosylation, free radical formation and pericyte death.11 These changes ultimately lead to vascular dilation, increased capillary hydrostatic pressure and microaneurysm formation. Duration and control of diabetes mellitus are of major significance in the development of DMO.
Previously, DMO was defined as CSMO or not, and focal laser treatment was initiated only for CSMO. More recently, DMO has been subcategorised into two main categories:12
Focal diabetic macular oedema (fDMO).
Diffuse diabetic macular oedema (dDMO).
The term centre-involving diabetic macular oedema (ciDMO) is also now commonly used to describe DMO in which the central macula is involved.
Age-related macular degeneration (AMD)13
The pathophysiology of this condition is covered in the separate related article. The prime pathological process seems to be degeneration of RPE cells, leading to a progressive irreversible degeneration of photoreceptors.
Continue reading below
How common is macular oedema? (Epidemiology)
CMO post-surgery: CMO occurs in only about 0.1-1.3% of cases of uncomplicated cataract surgery in a very mild form (in the past, with older techniques, up to 20% of patients developed CMO and the figure can be this high in more complex cases or where there is associated uveitis).7
Other causes of CMO: the incidence of other causes of CMO varies according to the specific pathology.
CSMO/ciDMO: when diabetes is undiagnosed and untreated, there is a 25-30% chance of developing vision-threatening DMO. This drops by half in treated patients. It is more likely to occur in patients aged over 60 years with systemic vascular diseases such as hypertension.
'Wet' AMD: this tends to occur in patients aged over 60 and is the leading cause of severe sight impairment and partial sight impairment certification in the over-65s.
In the less developed world a different picture is seen. A 2006 study of 4,800 patients in rural India found that 3.36% had bilateral severe sight impairment, about 75% of which were due to cataracts. Macular oedema accounted for 3.79% of cases.14
Symptoms of macular oedema (presentation)15
History
The exact presentation will vary with the underlying pathology but patients will complain of painless impairment of central vision.
There may be a scotoma (a black spot within the field of vision).
Visual acuity is impaired, typically in the region of 6/12 to 6/60.
Patients with Irvine-Gass syndrome usually experience a gradual blurring, with good initial vision.
Patients with AMD may complain of visual distortion (metamorphopsia), particularly of straight lines.
Some patients develop blue-yellow colour blindness.
There may be loss of contrast sensitivity.
Some patients note that colours in the central vision appear 'washed out'.
CSMO can be asymptomatic and only picked up in the diabetic screening clinic/unit.
Examination
The normal macula: what am I looking for?
The macula lies about 2 disc diameters temporally to the disc itself. It may be seen as a slightly darker area than the surrounding retina (more so in darker people) with blood vessels arching above and beneath it but not over it. Careful examination will reveal the foveal reflex, a small yellow-white shiny reflection in the centre of the macula - this is the fovea centralis.
Identifying macular oedema
It is not possible to diagnose macular oedema without the help of a slit lamp. A clue might be the loss of the foveal reflex compared to the fellow eye. In 'wet' AMD, there may also be an associated bleed, seen as a well-demarcated deep-red patch over the macular area. Even if the view is limited, the history and an abnormal Amsler grid will raise suspicions and should prompt referral.
Using an Amsler grid
This is a useful simple tool that can help screen for macular disease. The patient can use it at home to monitor progression.
It consists of a piece of paper on which a 10 cm x 10 cm box is printed, subdivided into 20 x 20 smaller squares and with a black dot in the centre. The patient is asked to wear any corrective lenses that they usually wear, hold the chart 33 cm away (arm's length) and cover one eye. They must then fix their gaze on the central dot. They are asked if they can see the four corners of the box. They are then told to comment on how straight the lines of the grid are and to draw over them as they see them in the parts that seem curved. Finally, they are asked to outline any areas missing within the box.
This should be repeated for the fellow eye. This gives a reasonable indication of macular function.
Continue reading below
Differential diagnosis15
Once macular oedema is confirmed, the cause is likely to be one of those outlined above. In the young (typically type A personality male) patient, it could also be confused with central serous chorioretinopathy, a generally self-limiting condition which typically arises in times of acute stress, whereby there is a localised accumulation of fluid within the retina.
Diagnosing macular oedema (investigations)
Macular oedema is usually diagnosed on slit-lamp examination.
Using a slit lamp, clinically significant foveal oedema and retinal thickening can be seen as a loss of foveal reflex; this is best seen using green light. Subclinical foveal oedema is better seen through retinal imaging.
In DMO, macular thickening with or without hard exudates (consisting of lipoproteins) may be seen.
CSMO is still extensively referred to in the literature and is defined as retinal thickening within 500 µm of the macular centre, hard exudates within 500 µm of the macular centre with adjacent thickening, or one or more disc diameters of retinal thickening, within one disc diameter of the macular centre. More recently the term centre-involving diabetic macular oedema (ciDMO) has been used to describe DMO in which the central macula is involved.
The diagnosis is often confirmed with optical coherence tomography (OCT), which is a sort of visual biopsy obtained in a similar fashion to an ultrasound scan but using light waves.16 It is a painless diagnostic imaging process performed in the outpatient setting, with the patient looking into the OCT machine for a few moments.
OCT is useful both to confirm diagnosis (newer software is now able to distinguish between different patterns of CMO associated with different underlying pathology) and to monitor progress. Fluorescein angiography may also be required to further refine the diagnosis and guide management.
Management of macular oedema10 16
The management of macular oedema depends on its aetiology and extent. Many causes of macular oedema respond to treatment of the underlying condition (eg, CMO associated with CMV retinitis is treated by managing the retinitis with antiviral agents).
Current treatment of CMO
Non-steroidal anti-inflammatory drugs (NSAIDS) - topical or systemic indomethacin decreases the production of prostaglandins. Ketorolac 0.5%, indomethacin 1%, and diclofenac 1% are used postoperatively for aphakic eyes and in other conditions where there is inflammation.
Corticosteroids - topical, periocular, systemic, intravitreal injection or implant corticosteroids inhibit prostaglandin and leukotriene production. Steroids, particularly intravitreal triamcinolone, help in uveitic macular oedema. Side-effects of steroid injection include glaucoma and cataract formation.
Dexamethasone intravitreal implants are used for the treatment of macular oedema following central retinal vein occlusion and for branch retinal vein occlusion when laser treatment has not worked, or is not possible. It suppresses inflammation in the eye by inhibiting oedema, fibrin deposition, capillary leakage, phagocytic migration, expression of vascular endothelial growth factor and the release of prostaglandins. The treatment is effective but the duration of improvement is typically limited to 2 to 3 months and the treatment may need to be repeated. There are only limited published data on long-term outcomes.17 18 19
Immunomodulators including methotrexate, mycophenolate mofetil, tacrolimus, azathioprine, and ciclosporin, as well as biologic agents, notably the anti-tumour necrosis factor-α monoclonal antibodies adalimumab and infliximab, may be useful in refractory cases of uveitic macular oedema, or enable the tapering of steroids.20
Carbonic anhydrase inhibitors - these alter ionic transport systems in the retinal pigment epithelial cells, moving fluid away from the intracellular spaces.
Laser photocoagulation - this uses a light source to coagulate retinal and retinal pigment epithelial cell tissue.21 It is thought that adjacent healthy RPE cells then replace necrotic cells. The other view it that a reduction of oxygen consumption in the outer retina allows diffusion of oxygen to the inner retina.
Anti-VEGF agents - pegaptanib, ranibizumab and bevacizumab act by decreasing vascular permeability from disrupted endothelial cells. Marked reduction in retinal thickness and fluid accumulation has been noted in various studies with a significant improvement in visual acuity with minimal side-effects. A National Institute for Health and Care Excellence (NICE) technology appraisal in 2013 recommended ranibizumab for visual impairment caused by CMO following venous occlusion.22
Pharmacological vitreolysis agents - enzymatic vitreolysis with agents such as chondroitinase, dispase, hyaluronidase, plasmin and microplasmin can induce posterior vitreous detachment to relieve traction on the retina.
Surgery - vitrectomy can help to relieve macula oedema refractory to medical therapy. It may also clear high levels of inflammatory mediators, although evidence remains inconclusive. Side-effects of vitrectomy include cataracts, retinal detachment, vitreous haemorrhage and a rise in intraocular pressure.
Current treatment of DMO
Treatment aims in DMO have changed from the maintenance or reduction in rate of visual loss, to reversal of the loss of visual acuity.
The current approach uses a combination of anti-VEGF agents, laser treatment and corticosteroids designed to address the multifactorial nature of the disease. Potential novel therapies targeting molecules other than VEGF are being developed and evaluated.
Steroids: NICE has recommended that fluocinolone or dexamethasone intravitreal implants are an option for treating visual impairment caused by diabetic macular oedema in adults.23 24
Anti-VEGF agents: Aflibercept and ranibizumab are currently NICE recommended treatments for diabetic macular oedema.25 26 NICE has also recommended faricimab or brolucizumab as alternative options to treatment with aflibercept or ranibizumab for diabetic macular oedema.27 28 These are all injectable options that are effective clinically and work similarly. They are initiated when the central retina has a thickness of 400 micrometres or more.
Laser photocoagulation:21 This has been the mainstay of treatment for a period of 25 years: the aim is to induce regression of new blood vessels and reduce central macular thickening. It is thought that the procedure works by reducing the release of vasoproliferative mediators by hypoxic retinal vessels and allowing easier direct diffusion of oxygen from the choroid blood supply.
Complications of macular oedema15
If macular oedema is prolonged, retinal thinning, scarring or a retinal hole can eventually ensue.
Prognosis15
CMO - this depends on the aetiology but in uncomplicated cases recovery is usually good after several months. 90-95% of these patients can expect a final visual acuity of 6/12 or better within 3-12 months of their operation. Severe cases can result in more permanent visual loss. In cases where irreversible processes have occurred (such as in central retinal vein occlusion), there may be little or no chance of recovery. Where there are inflammatory processes, the duration and severity of the condition will determine overall outcome.
DMO - improvement of visual function remains rare where deterioration of central vision has occurred. Historically, treatment has focused on arresting deterioration. With the introduction of combination therapies this is beginning to change.
'Wet' AMD - these patients tend to have a poor outcome, even with therapy (which is long, may be painful and is very expensive). Most experience a sudden and rapid deterioration in visual function that is irreversible. Vision never fully disappears and peripheral vision will remain normal in the absence of concurrent disease.
Prevention of macular oedema10
Pre-operative NSAIDs are sometimes given to high-risk patients in cataract surgery. Patients with diabetes need intensive steroid cover for cataract surgery, in order to minimise the risk of deterioration.
Good glycaemic control, blood pressure and cholesterol control may stall the development of retinopathy and DMO in patients with diabetes. Progression of the condition once it has developed can be arrested with laser and other treatment modalities. Regular retinal surveillance will help detect changes and enable intervention before vision deteriorates..
Patients with a known diagnosis of AMD are often issued with an Amsler grid in order to try to catch any macular changes early. AMD patients with 'wet' macular disease may be monitored in outpatients.13
AMD patients may also be advised to take multivitamin supplements to prevent progression of the disease in the other eye. The use of these supplements remains somewhat controversial and is more fully discussed in the separate Age-related Macular Degeneration article.
Dr Mary Lowth is an author or the original author of this leaflet.
Further reading and references
- Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al; Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185-222. doi: 10.1159/000458539. Epub 2017 Apr 20.
- Zhang W, Liu Y, Sang A; Efficacy and effectiveness of anti-VEGF or steroids monotherapy versus combination treatment for macular edema secondary to retinal vein occlusion: a systematic review and meta-analysis. BMC Ophthalmol. 2022 Dec 6;22(1):472. doi: 10.1186/s12886-022-02682-7.
- Song R, Jiang J, Wang H; Macular Edema and Visual Acuity Observation after Cataract Surgery in Patients with Diabetic Retinopathy. J Healthc Eng. 2022 Jan 25;2022:3311751. doi: 10.1155/2022/3311751. eCollection 2022.
- Daruich A, Matet A, Moulin A, et al; Mechanisms of macular edema: Beyond the surface. Prog Retin Eye Res. 2018 Mar;63:20-68. doi: 10.1016/j.preteyeres.2017.10.006. Epub 2017 Nov 7.
- Scholl S, Augustin A, Loewenstein A, et al; General pathophysiology of macular edema. Eur J Ophthalmol. 2011;21 Suppl 6:S10-9. doi: 10.5301/EJO.2010.6050.
- Cunha-Vaz J, Coscas G; Diagnosis of macular edema. Ophthalmologica. 2010;224 Suppl 1:2-7. doi: 10.1159/000315156. Epub 2010 Aug 18.
- Coscas G, Cunha-Vaz J, Soubrane G; Macular edema: definition and basic concepts. Dev Ophthalmol. 2010;47:1-9. doi: 10.1159/000320070. Epub 2010 Aug 10.
- Rotsos TG and Moschos MM; Cystoid macular edema. Clin Ophthalmol. 2008 Dec; 2(4): 919–930.
- Fardeau C, Champion E, Massamba N, et al; Uveitic macular edema. Eye (Lond). 2016 Oct;30(10):1277-1292. doi: 10.1038/eye.2016.115. Epub 2016 Jun 3.
- Moshirfar M, Milner D, Patel BC; Cataract Surgery.
- Chu CJ, Johnston RL, Buscombe C, et al; Risk Factors and Incidence of Macular Edema after Cataract Surgery: A Database Study of 81984 Eyes. Ophthalmology. 2016 Feb;123(2):316-323. doi: 10.1016/j.ophtha.2015.10.001. Epub 2015 Dec 8.
- Ruia S, Kaufman EJ; Macular Degeneration
- Fung TH, Patel B, Wilmot EG, et al; Diabetic retinopathy for the non-ophthalmologist. Clin Med (Lond). 2022 Mar;22(2):112-116. doi: 10.7861/clinmed.2021-0792.
- Shukla UV, Tripathy K; Diabetic Retinopathy.
- Jain A, Varshney N, Smith C; The evolving treatment options for diabetic macular edema. Int J Inflam. 2013;2013:689276. doi: 10.1155/2013/689276. Epub 2013 Sep 9.
- Thomas CJ, Mirza RG, Gill MK; Age-Related Macular Degeneration. Med Clin North Am. 2021 May;105(3):473-491. doi: 10.1016/j.mcna.2021.01.003. Epub 2021 Apr 2.
- Vijaya L, George R, Arvind H, et al; Prevalence and causes of blindness in the rural population of the Chennai Glaucoma Study. Br J Ophthalmol. 2006 Apr;90(4):407-10.
- Kohli P, Patel BC; Macular Edema.
- Amoaku WM, Ghanchi F, Bailey C, et al; Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye (Lond). 2020 Jun;34(Suppl 1):1-51. doi: 10.1038/s41433-020-0961-6.
- Dexamethasone intravitreal implant for the treatment of macular oedema caused by retinal vein occlusion; NICE Technology appraisal guidance, July 2011
- Kuppermann BD, Haller JA, Bandello F, et al; Onset and duration of visual acuity improvement after dexamethasone intravitreal implant in eyes with macular edema due to retinal vein occlusion. Retina. 2014 Sep;34(9):1743-9. doi: 10.1097/IAE.0000000000000167.
- Pelegrin L, de la Maza MS, Molins B, et al; Long-term evaluation of dexamethasone intravitreal implant in vitrectomized and non-vitrectomized eyes with macular edema secondary to non-infectious uveitis. Eye (Lond). 2015 Jul;29(7):943-50. doi: 10.1038/eye.2015.73. Epub 2015 May 22.
- Koronis S, Stavrakas P, Balidis M, et al; Update in treatment of uveitic macular edema. Drug Des Devel Ther. 2019 Feb 19;13:667-680. doi: 10.2147/DDDT.S166092. eCollection 2019.
- Everett LA, Paulus YM; Laser Therapy in the Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr Diab Rep. 2021 Sep 6;21(9):35. doi: 10.1007/s11892-021-01403-6.
- Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein occlusion; NICE Technology Appraisal, May 2013
- Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy; NICE Technology Appraisal Guidance, November 2013
- Dexamethasone intravitreal implant for treating diabetic macular oedema; NICE Technology appraisal guidance, September 2022
- Aflibercept for treating diabetic macular oedema; NICE Technology Appraisal, July 2015
- Ranibizumab for the treatment of diabetic macular oedema; NICE Technology Appraisal Guidance, February 2013 - last updated October 2023
- Faricimab for treating diabetic macular oedema; NICE Technology appraisal guidance, June 2022
- Brolucizumab for treating diabetic macular oedema; NICE Technology appraisal guidance, August 2022
Article History
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 5 Jan 2029
16 Jan 2024 | Latest version

Feeling unwell?
Assess your symptoms online for free