Gout
Peer reviewed by Dr Krishna Vakharia, MRCGPLast updated by Dr Colin Tidy, MRCGPLast updated 26 Jul 2022
Meets Patient’s editorial guidelines
- DownloadDownload
- Share
- Language
- Discussion
- Audio Version
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find the Gout article more useful, or one of our other health articles.
In this article:
Continue reading below
What is gout?1
Gout can be defined as a form of arthritis due to deposition of monosodium urate (MSU) crystals in the joints causing acute inflammation and eventual tissue damage.2
The duration and magnitude of hyperuricaemia is directly correlated with the likelihood of developing gouty arthritis and developing uric acid kidney stones. However, gout can occur in people with normal plasma urate levels and many people with hyperuricaemia never develop gout.
Hyperuricaemia is usually due to impaired renal excretion of urate. About 90% of people with hyperuricaemia are under-excreters of urate and about 10% are over-producers of urate. Some people are both under-excreters and over-producers of urate. In many people with hyperuricaemia, the cause is multifactorial.
Classification
Back to contentsThe classification of gout was updated in 2015 as a result of an American College of Rheumatology/European League Against Rheumatism collaborative initiative:3
1. Pattern of joint/bursa involvement during symptomatic (pain and/or swelling) episode(s) ever:
Joint(s) or bursa(e) other than ankle, midfoot or first metatarsophalangeal (MTP) joint (or their involvement only as part of a polyarticular presentation).
Ankle or midfoot joint(s) as monoarticular or part of an oligoarticular presentation without first MTP joint involvement.
MTP joint involvement as monoarticular or part of an oligoarticular presentation.
2. Characteristics of symptomatic episode(s) ever:
Great difficulty with walking or inability to use the affected joint(s) during a symptomatic episode ever (patient-reported).
Can't bear touch or pressure to the affected joint during a symptomatic episode ever (patient-reported).
Erythema overlying affected joint during a symptomatic episode ever (patient-reported or physician-observed).
3. Time course of symptomatic episode(s) ever. 'Typical symptomatic episode': presence (ever) of >2 of the following, irrespective of antiinflammatory treatment:
Time to maximal pain <24 hours.
Resolution of symptoms in ≤14 days.
Complete resolution (to baseline level) between symptomatic episodes.
4. Clinical evidence of tophus:
Appearance: draining or chalk-like subcutaneous nodule under transparent skin, often with overlying vascularity.
Classic locations: joints, ears, olecranon bursae, finger pads, tendons (eg, Achilles).
5. Serum urate level, off-treatment:
Categories are defined as:
<4 mg/dL (0.24 mmol/L).
4-5.9 mg/dL (0.24-0.36 mmol/L).
6-7.9 mg/dL (0.36-0.48 mmol/L).
8-9.9 mg/dL (0.48-0.60 mmol/L).
≥10 mg/dL (≥0.60 mmol/L)
Ideally, the serum urate level should be scored if tested at a time when the patient was not receiving urate-lowering therapy and it was >4 weeks from the start of an episode; if practicable, retest under those conditions. If serum urate level is ≥10 mg/dl, there is no need to retest.
6. Synovial fluid analysis:
Location: symptomatic (ever) joint or bursa.
Assessment should be performed by a trained observer.
7. Imaging evidence of urate deposition:
Double-contour sign on ultrasound, or urate deposition on dual-energy CT.
Location: symptomatic (ever) joint or bursa.
8. Imaging evidence of gout-related joint damage:
Appearance of gout-related erosion: cortical break with sclerotic margin and overhanging edge; excludes gull wing appearance.
Location: radiograph of hands and/or feet; excludes distal interphalangeal joints.
Continue reading below
How common is gout? (Epidemiology)1
Back to contentsGout is the most common inflammatory arthritis. It is increasing in prevalence worldwide.
Gout is more common in men and prevalence increases with age, plateauing at around 80 years of age. Gout is rare in people younger than 20 years old.
A study of UK general practice in 2012 found that the prevalence of gout was 2.49%, the incidence was 1.77 per 1,000 person years, and the overall ratio of men to women was 4.3:1.
Gout prevalence is reported at to be higher in Oceania, in North America and among indigenous populations such as Maori, Aboriginal and Inuit. The higher prevalence reported in North America may be attributed to higher rates in ethnic groups, such as African Americans and Filipinos, due to both increasing rates of hypertension and adoption of Western diet in these populations.
Risk factors4
Risk factors include:
Male gender.
Meat.
Seafood.
Alcohol (10 or more grams per day).
Diuretics.
Obesity.
Hypertension.
Coronary heart disease.
Diabetes mellitus.
Chronic kidney disease.
High triglycerides.
Heart failure.
Psoriasis.
Chemotherapy.
One large study found that excessive consumption of purine-rich foods and alcoholic drinks are independent risk factors for gout. The study also found that fructose and sugar-sweetened soft-drinks increase the risk of developing gout whereas dairy products, coffee and vitamin C appeared to be protective against the development of gout.5
Symptoms of gout1
Back to contentsThe European League Against Rheumatism (EULAR) guidelines for diagnosis suggest that the development of acute pain in a joint which becomes swollen, tender and erythematous and which reaches its crescendo over a 6- to 12-hour period is highly suggestive of crystal arthropathy, although not specifically of gout.4
50% of all attacks and 70% of first attacks affect the first MTP. Other sites often affected with pain and swelling are:
Knee
Midtarsal joints
Wrists
Ankles
Small hand joints
Elbows
The inflammation reaches its peak within 24 hours, often with fever and malaise.
Gout - podagra
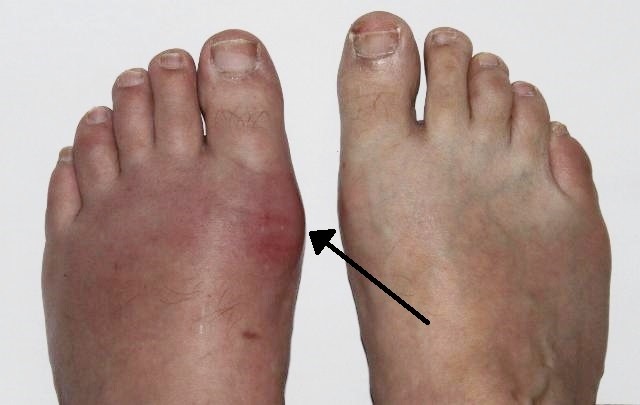
© Gonzosft, CC BY 3.0 DE, via Wikimedia Commons
By Gonzosft, CC BY 3.0 DE, via Wikimedia Commons
Some patients may only present with connective tissue tophi.6
Signs
There is florid synovitis and swelling and extreme tenderness with overlying erythema. Untreated, the attack resolves spontaneously over 5-15 days, usually with itching and desquamation of overlying skin.
Atypical attacks can occur with tenosynovitis, bursitis and cellulitis, with mild discomfort without swelling lasting a day or two.
Chronic tophaceous gout - in this condition large crystal deposits produce irregular firm nodules mainly around extensor surfaces of the fingers, hands, forearms, elbows, Achilles tendons and ears.
Typically, tophi are asymmetrical with a chalky appearance beneath the skin. Damage is usually found in the first MTP joints, midfoot, small finger joint and wrist, with restricted movement, crepitus and deformity.
The National Institute for Health and Care Excellence (NICE) recommends suspecting gout in people presenting with any of the following:7
Rapid onset (often overnight) of severe pain together with redness and swelling, in 1 or both first metatarsophalangeal (MTP) joints.
Tophi:8
Tophi are collections of monosodium urate crystals, which develop in soft tissues and appear as firm lumps under the skin.
Tophi generally develop around 10 years after the first attack of gout in untreated patients and are commonly found around the elbows, hands, and feet.
Tophi develop in and around the joints, leading to joint destruction and chronic (long-term, continuous) joint pain and stiffness.
Tophi contain a white pasty material and as they enlarge they work their way towards the skin surface to drain. Small sinus tracts (tunnels) may develop and secrete white pasty material. Alternatively a large blister may form, which ruptures leaving a continuously draining ulcer.8
Consider gout in people presenting with rapid onset (often overnight) of severe pain, redness or swelling in joints other than the first MTP joints (eg, midfoot, ankle, knee, hand, wrist, elbow).
Consider chronic gouty arthritis in people presenting with chronic inflammatory joint pain.
Continue reading below
Differential diagnosis
Back to contentsAcute attacks - septic arthritis and other forms of crystal-related synovitis.
Chronic tophaceous - rheumatoid arthritis, generalised nodal osteoarthritis, xanthomatosis with arthropathy, multicentric reticulohistiocytosis.
Assess the possibility of septic arthritis, calcium pyrophosphate crystal deposition and inflammatory arthritis in people presenting with a painful, red, swollen joint. If septic arthritis is suspected, refer immediately according to the local care pathway.7
Investigations4
Back to contentsThe EULAR guidelines recommend the following evidence-based approach:
For typical presentations such as inflammation of the first MTP joint (also known as podagra) with hyperuricaemia, a clinical diagnosis can be made with reasonable accuracy but is not definitive unless the presence of uric acid crystals can be demonstrated.
Demonstration of MSU crystals in synovial fluid or tophi confirms the diagnosis of gout.
Since gout can present atypically, an opportunity should be taken to examine all samples of synovial fluid aspirated from joints for MSU crystals, even if not inflamed at the time.
Gram staining and culture of synovial fluid should be arranged, even if MSU crystals are found, since gout and sepsis can co-exist.
Although a raised serum uric acid (SUA) level is an important risk factor for gout, the use of SUA as a diagnostic test is limited. It can be normal during acute gout, whilst patients with hyperuricaemia may never develop an attack. Studies suggest that the cut-off point above which a level can be considered raised is 360 μmol/L.
Renal uric acid secretion (as detected by a 24-hour urine sample) may be helpful in diagnosis, particularly in patients with a family history of young-onset gout, patients whose first attack of gout was under the age of 25 years and patients with renal stones. Such patients are likely to be over-producers of uric acid.
Radiology:
X-Rays may be useful in chronic gout, when punched-out lesions, areas of sclerosis and, in the latter stages, tophi may be seen. The first lesions usually occur in and around the first MTP joint.
Ultrasound, dual-energy CT and MRI are among the current imaging modalities that can identify urate deposition, structural joint damage, and joint inflammation in gout.9
Fasting glucose and lipids should be performed to rule out hyperglycaemia and hyperlipidaemia, as gout is commonly associated with metabolic syndrome.
NICE recommends:7
Measure the serum urate level in people with symptoms and signs of gout to confirm the clinical diagnosis (serum urate level of 360 micromol/litre [6 mg/dL] or more). If serum urate level is below 360 micromol/L (6 mg/dl) during a flare and gout is strongly suspected, repeat the serum urate level measurement at least two weeks after the flare has settled.
Consider joint aspiration and microscopy of synovial fluid if a diagnosis of gout remains uncertain or unconfirmed.
If joint aspiration cannot be carried out or the diagnosis of gout remains uncertain, consider imaging the affected joints with X-ray, ultrasound or dual-energy CT.
Gout treatment and management1 7
Back to contentsGeneral points
An ice pack may be useful, as may rest. The joint should be elevated and trauma avoided.
Pharmacological therapeutic options include:
Non-steroidal anti-inflammatory drugs (NSAIDs).
Colchicine.
Corticosteroids.
Other primarily analgesic compounds.
The choice for a particular patient will depend on:
Contra-indications.
The gap between onset of symptoms and the start of treatment.
Risks versus benefits.
EULAR guidelines recommend colchicine and/or NSAIDs as the first-line option for acute gout.4
The opportunity should be taken to discuss lifestyle issues such as , exercise, diet (such as red meat intake), alcohol consumption and fluid intake.
Canakinumab
Canakinumab is a recombinant monoclonal antibody active as an inhibitor of proinflammatory cytokine IL-1. It is licensed for use in people with gouty arthritis whose condition has not responded adequately to treatment with NSAIDs or colchicine, or in those with contra-indications or intolerances to them, and in whom repeated courses of corticosteroids are inappropriate.10
NSAIDs
NSAIDs are the first-line treatment. The sooner medication is started, the more rapid the response.11 Consider giving the patient a stock to keep at home.
Indometacin has been traditionally used first-line in the past but there is no convincing evidence to support the use of any particular NSAID.12 Eight drugs are licensed for use in gout. Diclofenac, naproxen and indometacin are generally preferred.
For patients with a high risk of gastrointestinal adverse events, use a gastro-protective agent, simple analgesia, or colchicine. Tailor the dose to the needs of the patient, bearing in mind age, comorbidity and interactions with other drugs. Aim for the highest tolerable licensed dose but be aware of the Commission on Human Medicine's guidance to use NSAIDs for the shortest possible time in view of cardiovascular risk.
Colchicine
Colchicine is an effective treatment for gout. The British National Formulary (BNF) recommends 500 micrograms 2-4 times daily until symptoms are relieved - maximum 6 mg per course; the course is not to be repeated within three days. In practice, the maximum dose is often limited by the development of toxicity symptoms (nausea, vomiting, diarrhoea).10
Colchicine is particularly appropriate when NSAIDs are poorly tolerated, in patients with heart failure and in those who are on anticoagulants.13 14
The drug can be effective at lower doses.15 Titrate up to the maximum licensed dose, according to response.
Corticosteroids
These can be given orally, intramuscularly, intravenously or intra-articularly.12 However a Cochrane review found inconclusive evidence for the efficacy and effectiveness of systemic corticosteroids in the treatment of acute gout.16
Intra-articular administration of long-acting steroids has been shown, in small trials, to be safe and effective.4 However, further work is needed to clarify effectiveness. It can be paired with aspiration of the joint, making it convenient to both aid diagnosis and to manage the condition. It is particularly useful for those patients with a severe monoarthritis and contra-indications to NSAIDs and colchicine. It is also useful as it is associated with minimal adverse effects and a lower risk of drug interactions. It should not be undertaken if septic arthritis is suspected.
Analgesics
These are useful where all other drug groups are contra-indicated or as an adjunct for pain relief. Start with paracetamol, with or without codeine, taken regularly rather than only as required.
Prophylactic drugs
Allopurinol
Allopurinol, a xanthine oxidase inhibitor, is considered one of the most effective urate-lowering drugs and is frequently used in the treatment of chronic gout. However, a Cochrane review found moderate-quality evidence of little or no difference in achieving target serum urate when allopurinol was compared with benzbromarone. Allopurinol seemed more successful than placebo and may be less successful than febuxostat in achieving a target serum urate level based on low- to moderate-quality evidence.17
Febuxostat10
This is recommended by NICE as an option for the management of chronic hyperuricaemia in gout. The Medicines and Healthcare products Regulatory Agency (MHRA) issued advice in 2012 that febuxostat can cause serious hypersensitivity reactions, including Stevens-Johnson syndrome and acute anaphylactic shock. MHRA provided further advice for prescribing febuxostat in 2019 because of an increased risk of cardiovascular death and all-cause mortality in a clinical trial in patients with a history of major cardiovascular disease. Therefore it has been advised to avoid treatment with febuxostat in patients with pre-existing major cardiovascular disease, unless no other therapy options are appropriate.
Uricosurics18
Uricosurics act by increasing renal urate excretion, mediated by selective inhibition of organic anion transporters present in the proximal renal tubular cells. All uricosurics have an increased risk of precipitation of urate stones.
The following management of gout is recommended by NICE:7
Managing gout flares
Offer a non-steroidal anti-inflammatory drug (NSAID), colchicine or a short course of an oral corticosteroid for first-line treatment of a gout flare, taking into account the person's comorbidities, co-prescriptions and preferences. Consider adding a proton pump inhibitor for people with gout who are taking an NSAID to treat a gout flare.
Consider an intra-articular or intramuscular corticosteroid injection to treat a gout flare if NSAIDs and colchicine are contra-indicated, not tolerated or ineffective.
Do not offer an interleukin-1 (IL-1) inhibitor to treat a gout flare unless NSAIDs, colchicine and corticosteroids are contra-indicated, not tolerated or ineffective. Refer the person to a rheumatology service before prescribing an IL-1 inhibitor.
Advise people with gout that applying ice packs to the affected joint (cold therapy) in addition to taking prescribed medicine may help alleviate pain.
Follow-up after a gout flare
Consider a follow-up appointment after a gout flare has settled to:
Measure the serum urate level.
Provide information about gout and how to self-manage and reduce the risk of future flares.
Assess lifestyle and comorbidities (including cardiovascular risk factors and chronic kidney disease).
Review medications and discuss the risks and benefits of long-term urate-lowering therapy (ULT).
Diet and lifestyle
There is not enough evidence to show that any specific diet prevents flares or lowers serum urate levels. Advise to follow a healthy, balanced diet.
Excess body weight or obesity, or excessive alcohol consumption, may exacerbate gout flares and symptoms.
Long-term management of gout
Management of gout with urate-lowering therapies:
Offer ULT, using a treat-to-target strategy (see below), to people with gout who have:
Multiple or troublesome flares.
CKD stages 3 to 5 (glomerular filtration rate [GFR] categories G3 to G5).
Diuretic therapy.
Tophi.
Chronic gouty arthritis.
Discuss the option of ULT, using a treat-to-target strategy, with people who have had a first or subsequent gout flare who are not within these groups.
Ensure people understand that ULT is usually continued after the target serum urate level is reached, and is typically a lifelong treatment.
Start ULT at least 2-4 weeks after a gout flare has settled. If flares are more frequent, ULT can be started during a flare.
Treat-to-target strategy: start with a low dose of ULT and use monthly serum urate levels to guide dose increases, as tolerated, until the target serum urate level is reached.
Target serum urate level:
Aim for a target serum urate level below 360 micromol/L (6 mg/dL).
Consider a lower target serum urate level below 300 micromol/L (5 mg/dL) for people with gout who:
Have tophi or chronic gouty arthritis
Continue to have ongoing frequent flares despite having a serum urate level below 360 micromol/L (6 mg/dL).
Urate-lowering therapies
Offer either allopurinol or febuxostat as first-line treatment when starting treat-to-target ULT.
Offer allopurinol as first-line treatment to people with gout who have major cardiovascular disease (for example, previous myocardial infarction or stroke, or unstable angina).
Consider switching to second-line treatment with allopurinol or febuxostat if the target serum urate level is not reached or first-line treatment is not tolerated.
Preventing gout flares when starting or titrating urate-lowering therapy
For people who choose to have treatment to prevent gout flares when starting or titrating ULT, offer colchicine while the target serum urate level is being reached. If colchicine is contra-indicated, not tolerated or ineffective, consider a low-dose NSAID or low-dose oral corticosteroid.
Consider adding a proton pump inhibitor for people with gout who are taking an NSAID or a corticosteroid to prevent gout flares when starting or titrating ULT.
Do not offer an IL-1 inhibitor when starting or titrating ULT to prevent gout flares unless colchicine, NSAIDs and corticosteroids are contra-indicated, not tolerated or ineffective. Refer the person to a rheumatology service before prescribing an IL-1 inhibitor.
Monitoring serum urate level
Consider annual monitoring of serum urate level in people with gout who are continuing ULT after reaching their target serum urate level.
Referral to specialist services
Consider referring a person with gout to a rheumatology service if:
The diagnosis of gout is uncertain.
Treatment is contra-indicated, not tolerated or ineffective.
They have CKD stages 3b to 5 (GFR categories G3b to G5).
They have had an organ transplant.
Complications1
Back to contentsRenal disease:
Chronic urate nephropathy results from widespread deposition of urate crystals in the interstitium of medulla and pyramids, causing inflammation and fibrosis.
Gout patients who have a 24-hour urinary excretion of uric acid above 780 mmol/L have a 50% risk of developing urate and oxalate kidney stones. Those with a measured urate excretion greater than 800 mg per 24 hours may benefit from allopurinol prophylaxis to prevent urate nephropathy.
Severe degenerative arthritis.
Secondary infections.
Recurrent painful episodes.
Carpal tunnel syndrome (rare).
Nerve or spinal cord impingement.
Prognosis1
Back to contentsFirst acute attacks usually completely resolve within 3-10 days. Attacks have been reported to recur in 62% of people within a year. Recurrent acute episodes and the development of chronic gout lead to progressive joint damage, pain and disability. Serum uric acid levels greater than 360 µmol/L are associated with increased risk for recurrent gout attacks.
Further reading and references
- Clebak KT, Morrison A, Croad JR; Gout: Rapid Evidence Review. Am Fam Physician. 2020 Nov 1;102(9):533-538.
- Abhishek A, Roddy E, Doherty M; Gout - a guide for the general and acute physicians. Clin Med (Lond). 2017 Feb;17(1):54-59. doi: 10.7861/clinmedicine.17-1-54.
- Li Q, Li X, Wang J, et al; Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements. BMJ Open. 2019 Aug 24;9(8):e026677. doi: 10.1136/bmjopen-2018-026677.
- Gout; NICE CKS, February 2018 (UK access only)
- Rheumatology AF, Rheumatologist PK; The management of gout. Aust Prescr. 2016 Aug;39(4):119-122. Epub 2016 Aug 1.
- Neogi T, Jansen TL, Dalbeth N, et al; 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015 Oct;67(10):2557-68. doi: 10.1002/art.39254.
- 2016 updated EULAR evidence-based recommendations for the management of gout; European League Against Rheumatism (2016)
- Roddy E, Choi HK; Epidemiology of gout. Rheum Dis Clin North Am. 2014 May;40(2):155-75. doi: 10.1016/j.rdc.2014.01.001. Epub 2014 Feb 19.
- Thissen CA, Frank J, Lucker GP; Tophi as first clinical sign of gout. Int J Dermatol. 2008 Nov;47 Suppl 1:49-51. doi: 10.1111/j.1365-4632.2008.03961.x.
- Gout: diagnosis and management; NICE guideline (June 2022)
- Gout; DermNet NZ
- Vargas-Santos AB, Taylor WJ, Neogi T; Gout Classification Criteria: Update and Implications. Curr Rheumatol Rep. 2016 Jul;18(7):46. doi: 10.1007/s11926-016-0594-8.
- British National Formulary (BNF); NICE Evidence Services (UK access only)
- Burns CM, Wortmann RL; Latest evidence on gout management: what the clinician needs to know. Ther Adv Chronic Dis. 2012 Nov;3(6):271-86. doi: 10.1177/2040622312462056.
- Laubscher T, Dumont Z, Regier L, et al; Taking the stress out of managing gout. Can Fam Physician. 2009 Dec;55(12):1209-12.
- Darling EK, McDonald H; A meta-analysis of the efficacy of ocular prophylactic agents used for the prevention of gonococcal and chlamydial ophthalmia neonatorum. J Midwifery Womens Health. 2010 Jul;55(4):319-27.
- Wertheimer AI, Davis MW, Lauterio TJ; A new perspective on the pharmacoeconomics of colchicine. Curr Med Res Opin. 2011 May;27(5):931-7. doi: 10.1185/03007995.2011.563284. Epub 2011 Mar 3.
- Richette P, Bardin T; Colchicine for the treatment of gout. Expert Opin Pharmacother. 2010 Dec;11(17):2933-8. doi: 10.1517/14656566.2010.529432.
- Janssens HJ, Lucassen PL, Van de Laar FA, et al; Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008 Apr 16;(2):CD005521. doi: 10.1002/14651858.CD005521.pub2.
- Seth R, Kydd AS, Buchbinder R, et al; Allopurinol for chronic gout. Cochrane Database Syst Rev. 2014 Oct 14;(10):CD006077. doi: 10.1002/14651858.CD006077.pub3.
- Sattui SE, Gaffo AL; Treatment of hyperuricemia in gout: current therapeutic options, latest developments and clinical implications. Ther Adv Musculoskelet Dis. 2016 Aug;8(4):145-59. doi: 10.1177/1759720X16646703. Epub 2016 May 2.
Continue reading below
Article history
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 25 Jul 2027
26 Jul 2022 | Latest version

Ask, share, connect.
Browse discussions, ask questions, and share experiences across hundreds of health topics.

Feeling unwell?
Assess your symptoms online for free