Uveitis
Peer reviewed by Dr Hayley Willacy, FRCGP Last updated by Dr Laurence KnottLast updated 19 Feb 2021
Meets Patient’s editorial guidelines
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find the Uveitis article more useful, or one of our other health articles.
In this article:
Uveitis is inflammation of the uveal tract, with or without inflammation of neighbouring structures (eg, the retina or vitreous). The term covers a diverse group of conditions leading to the common end-point of intraocular inflammation, and which have been estimated to cause approximately 10% of severe visual impairment. This makes uveitis one of the leading causes of preventable severe visual loss in developed countries1 .
See the separate Chorioretinal Inflammation and Choroidal Disorders articles for additional detail on posterior uveitis.
Continue reading below
Anatomy of the uveal tract
The uveal tract is the pigmented middle layer of the three concentric layers that make up the eye, lying between the sclera (superficial to it) and the retina (deep to it). It consists of the iris, ciliary body and choroid. The name comes from the Latin uva, meaning grape; it is possibly a reference to its purplish colour, wrinkled appearance and grape-like size and shape.
Human eye schematic
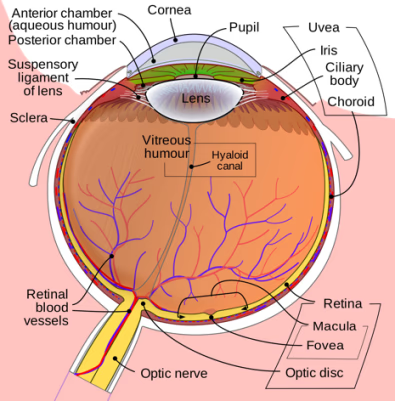
Attribution: by Rhcastilhos, via Wikimedia Commons
Classification of uveitis2 3
Anatomical
Uveitis may be unilateral or bilateral. It is classified by anatomical location of the inflammation, by inflammatory appearance and by chronicity:
Unilateral conditions are more commonly acute and can be infectious.
Bilateral conditions are likely due to chronic, systemic conditions.
Uveitis may be anterior, intermediate, posterior or panuveitis. More anatomically descriptive terms have been used in the past and persist in scientific literature and usage.
Anterior uveitis describes inflammation of the iris. It is also referred to as iritis, iridocyclitis (if there is also ciliary body involvement) or anterior cyclitis (if only the anterior portion of the ciliary body is affected).
Intermediate uveitis affects the vitreous and posterior part of the ciliary body. It is also referred to as pars planitis or posterior cyclitis, or as hyalitis when the inflammation involves only the anterior portion of the vitreous.
Posterior uveitis describes inflammation of the choroid. It is also referred to as choroiditis, or as chorioretinitis if the retina is also involved. It may also affect the retinal blood vessels, giving rise to retinal vasculitis. Posterior uveitis is further defined as being focal, multifocal or diffuse, depending on the nature of the inflammatory lesions seen on the fundus.
Panuveitis describes inflammation throughout the uveal tract.
By duration and course4
Acute conditions typically have an abrupt onset lasting several weeks. If left untreated, an acute condition can develop into a chronic cellular response.
Chronic uveitis is active uveitis that persists longer than three months. It is associated with a high incidence of vision-threatening complications, such as cataract, macular oedema and glaucoma, which may cause irreversible visual loss3 .
Granulomatous inflammation vs non-granulomatous5
Keratic precipitates (KPs) are clusters of white blood cells found on the posterior (endothelial) part of the cornea, resembling little white spots. Those which are larger and greasy looking are referred to as mutton-fat KPs. Uveitis is classified by the morphology of the KPs into non-granulomatous (small KPs) and granulomatous (mutton-fat KPs):
Non-granulomatous uveitis is more common. It is usually anterior and has an acute onset accompanied by a cellular reaction in the anterior chamber, involving smaller cell types (lymphocytes) than those in granulomatous inflammation. It is most commonly idiopathic or due to HLA-B27 involvement.
Granulomatous uveitis is usually chronic. There are large inflammatory cells in the anterior chamber. It is often associated with systemic conditions and autoimmune reaction, or arises from the host's immune response to a systemic infectious process, such as syphilis, Lyme disease, tuberculosis or herpetic viral infection.
Continue reading below
Aetiology
The various forms of uveitis represent the common end result of multiple underlying causes of ocular inflammation. These include3 :
Inflammatory - due to autoimmune disease.
Infectious - caused by known ocular and systemic pathogens.
Infiltrative - secondary to invasive neoplastic processes (sometimes referred to as masquerade syndromes). Intraocular lymphoma may present as a chronic uveitis in older patients. Intraocular tumours may also occasionally present with posterior uveitis.
Trauma - a common cause of anterior uveitis. Sympathetic ophthalmia (sometimes referred to as sympathetic ophthalmitis) is a rare form of bilateral panuveitis in response to trauma or surgery to one of the eyes. See also the separate Sympathetic Ophthalmia article.
Iatrogenic - caused by surgery, inadvertent trauma, or medication (eg, rifabutin, cidofovir)6 .
Inherited - secondary to metabolic or dystrophic disease.
Ischaemic - caused by impaired circulation.
Idiopathic - when evaluation has failed to find an underlying cause. Most uveitis, particularly anterior uveitis, is idiopathic.
Immunosuppression causes a particular risk of infection-related uveitis.
In Britain, sarcoidosis is the most common systemic disease that presents as chronic uveitis. In Japan, Behçet's disease is the most common systemic disease associated with chronic uveitis and, in other parts of the world, it may be tuberculosis7 .
Some ocular syndromes (HLA-B27-associated acute anterior uveitis) can give rise to anterior or posterior uveitis8 .
Pathophysiology3 9
Uveitis is the eye's response to a wide range of intraocular inflammatory diseases of infectious, traumatic, genetic or autoimmune aetiology. The end pathology results from the presence of inflammatory cells and the sustained production of cytotoxic cytokines and other immune regulatory proteins in the eye.
Trauma-related uveitis may result from a combination of microbial contamination and accumulation of necrotic products at the site of injury, stimulating an inflammatory response.
Infection-related uveitis may produce inflammation through the immune reaction directed against foreign antigens, which injure the uveal tract vessels and cells.
Autoimmune uveitis may result from immune complex deposition within the uveal tract.
Recent research has introduced the concept of 'molecular mimicry', where an infectious agent cross-reacts with ocular proteins. This triggers an inflammatory response.
In one study of 3,000 in the UK, the distribution in aetiology was as follows10 :
Fuchs' heterochromic uveitis (11.5% of total).
Sarcoidosis-related uveitis (9.7%).
Idiopathic intermediate uveitis (7.9%).
Idiopathic acute anterior uveitis (7.0%).
Toxoplasmosis (6.9%).
Continue reading below
Epidemiology1 4 5
Anterior uveitis is the most common form in the UK.
The prevalence of uveitis is variously given as 25-50 per 100,000 persons, with the mean onset at 30.7 years of age.
Most people who develop uveitis are aged 20-50 years.
Roughly 5% to 10% of these cases occur in children under the age of 16.
The epidemiology of uveitis varies with geographical location. Finland has one of the highest incidences of uveitis and this is thought to be because of the high frequency of HLA-B27 spondylopathy amongst the population.
Racial predisposition is related to the patient's underlying systemic disease (eg, HLA-B27 positivity in Caucasians, Behçet's disease in individuals of Mediterranean origin).
The prevalence of each underlying aetiology varies with age group, race and gender. For example:
Children: juvenile rheumatoid arthritis, toxocariasis.
Young adults: Behçet's disease, human leukocyte-associated antigen B27-associated uveitis, Fuchs' uveitis.
Older adults: Vogt-Koyanagi-Harada (VKH) syndrome, herpes zoster ophthalmicus and, in the developing world, tuberculosis and leprosy.
Males: ankylosing spondylitis, reactive arthritis, Behçet's disease, sympathetic ophthalmia.
Females: Rheumatoid arthritis, juvenile rheumatoid arthritis.
Presentation4 11 12
Clinical features vary depending on the location of the inflammation. Symptoms may develop over hours or days (acute uveitis), or onset may be gradual (chronic uveitis).
Symptoms11
Acute anterior uveitis
Usually unilateral.
Pain, redness and photophobia are typical.
Eye pain is often worse when trying to read.
Progressive - occurs over a few hours/days.
Blurred vision.
There may be excess tear production.
Associated headache is common.
Not all symptoms may be present at the start of an attack.
Chronic anterior uveitis
Recurrent episodes, with less acute symptoms.
Blurred vision and mild redness are common, often with little pain or photophobia, except during an acute episode.
Patients may find that one symptom predominates (typically this is blurred vision). They tend to become good at spotting this early.
Intermediate uveitis
Painless floaters and decreased vision (as for posterior uveitis).
Minimal external signs of inflammation (such as redness or pain).
Posterior uveitis
Gradual visual loss.
Usually bilateral.
Blurred vision and floaters.
Occasional photophobia; however, general absence of anterior symptoms (pain, redness and photophobia).
The presence of symptoms of posterior uveitis WITH pain suggests:
Anterior chamber involvement (panuveitis).
Bacterial endophthalmitis.
Posterior scleritis.
Orbital inflammatory disease.
Sudden bilateral loss of vision suggests VKH syndrome or sympathetic ophthalmia.
Panuveitis
Presents with any combination of the above symptoms.
NB: some types of uveitis are more insidious and may be asymptomatic (eg, that associated with juvenile idiopathic arthritis).
Examination
Fully dilated eye examination, including slit-lamp examination, is required to look for signs of posterior disease. The structures of the eye should be examined for diagnostic features and for features which may point to the underlying cause. Intraocular pressures (IOPs) should also be checked.
Visual acuity
Should be checked, may be reduced and, if so, is an important factor in management.
External eye
Lids, lashes and lacrimal ducts are normal.
Conjunctiva: typically 360° perilimbal injection, more intense close to the limbus; this is the reverse of the pattern seen in conjunctivitis, in which the most severe inflammation occurs further from the limbus.
Visual acuity may be decreased.
Extraocular movement: generally normal.
Pupils
There may be direct photophobia.
There may be consensual photophobia (typical of iritis: photophobia due to more superficial causes is typically direct but not consensual).
Miosis is common.
Epithelium and stroma
Look for abrasions, oedema, ulcers, foreign bodies.
Cornea
Look for:
KPs - inflammatory cells clumped together on the posterior (endothelial) part of the cornea as little white spots. KPs are characteristic of uveitis. When KPs appear large and granular or greasy, the uveitis is described as granulomatous.
Ciliary flush, a violaceous ring around the cornea, suggests intraocular inflammation.
Corneal oedema may be seen.
Lens
Lens changes may occur, most commonly posterior subcapsular opacity secondary to steroid use. These are not specific for uveitis.
Anterior chamber
In uveitis, severely inflamed vessels leak protein which clouds the normally clear aqueous. This looks hazy with the slit lamp. If severe, it disperses the light beam, causing flare.
White or red blood cells may be observed: the presence of inflammatory cells in the anterior chamber suggests inflammation of the iris and ciliary body.
Blood cells: grading of blood cells in the anterior chamber is as follows:
0 - none.
1+ - faint (barely detectable).
2+ - moderate (clear iris and lens details).
3+ - moderate (hazy iris and lens details).
4+ - intense (fibrin deposits, coagulated aqueous).
Hypopyon may be present if there is significant inflammation. This is highly suggestive of diseases associated with HLA-B27, Behçet's disease or, less commonly, infectious uveitis.
Iris
There may be anterior or posterior synechiae on the iris as a result of inflammation. These have the potential to lead to secondary glaucoma.
Iris atrophy is a diagnostic feature of herpetic uveitis. Herpes simplex causes diffuse atrophy while herpes zoster typically causes sectoral atrophy.
Iris nodules may be seen in sarcoidosis, tuberculosis, VKH syndrome, sympathetic ophthalmia and syphilis.
Vitreous
Inflammatory cells in the vitreous, which may be clumped, suggest posterior disease.
Retina and macula
Associated retinitis causes a yellow-white appearance and poorly defined edges, often associated with haemorrhage and exudation.
Retinal vasculitis is usually seen in retinitis and may be seen in granulomatosis with polyangiitis, systemic lupus erythematosus, and viral retinitis including herpetic infections.
Inflammation may lead to cystoid macular oedema, neovascularisation and macular holes.
Optic disc
Disc inflammation can occur even without other signs of uveitis. It usually appears as papillitis or disc oedema, neovascularisation, infiltration and cupping.
Sarcoidosis and leukaemia can infiltrate the disc tissue, producing an appearance similar to papillitis.
Intraocular pressure
IOP is most often decreased owing to impaired production of aqueous by the non-pigmented ciliary body epithelium. However, in some infective conditions (eg, herpetic uveitis, toxoplasmosis) it is increased due to the accumulation of inflammatory material and debris in the trabecular meshwork, trabeculitis, obstruction of venous return, and steroid therapy.
NB: if the history or examination suggests systemic symptoms, a further full physical examination is needed3 4 .
Examination findings by anatomical type
Anterior uveitis
Visual acuity is often reduced.
The pupil may be abnormally shaped or of a different size to the unaffected eye.
Direct photophobia and consensual photophobia (pain on shining a light into the unaffected eye) may be demonstrated.
Circumlimbal injection (injection around the corneal limbus) is characteristic. This can be fairly localised but, as the uveitis progresses, the entire conjunctiva may appear red.
The main signs are seen in the anterior chamber:
The characteristic sign is the presence of cells in the aqueous humour. As you shine the slit-lamp beam through the anterior chamber, the appearance is of a shaft of light shining through darkness with bits of dust floating through it.
The severity of the uveitis is graded by the number of cells seen, ranging from 0 (no cells seen) to +4 (>50 cells seen).
The aqueous humour may become cloudy, with 'flare'. This appears rather like a shaft of light shining through a darkened, smoky room. Flare is graded from 0 (no flare) to +4 (fibrin deposition).
Other findings may include synechiae and keratic precipitates.
Intermediate uveitis13
Intermediate uveitis involves the anterior vitreous, ciliary body and peripheral retina.
Inflammatory cells in the vitreous are best seen with a slit lamp in the dilated eye.
Pars planitis refers to a subset of intermediate uveitis where clumps of white cells form globular yellow-white 'snowballs' in the inferior peripheral vitreous, and yellow-white exudates, band to form 'snowbanks'.
Posterior uveitis
Inflammatory lesions may be seen on the retina or choroid. They may look yellow when fresh; older ones have a more distinct edge and a whitish appearance.
Inflammation of the retinal blood vessels (retinal vasculitis) may occur.
Oedema of the optic nerve may occur.
The inflammation may 'spill over' anteriorly so that there are inflammatory cells in the vitreous.
Scoring systems in uveitis
Various systems based on scoring a number of presenting clinical features have been proposed to assist in diagnosis and to standardise research findings2 14 15 . These include:
Location of inflammation.
Anterior chamber cells.
Anterior chamber flare.
Vitreous cells (present or absent).
Vitreous haze.
Differential diagnosis
Any cause of a red eye or visual disturbance should be included in the differential diagnosis.
Investigations
A first episode of mild, unilateral non-granulomatous acute uveitis can be diagnosed by history and clinical examination alone. Laboratory investigation is not usually helpful if there is trauma or a known systemic disease, or if the history and examination do not suggest systemic disease.
Imaging may be helpful where there is posterior disease, in order to assess site or severity of posterior inflammation. Fundus fluorescein angiography and optical coherence tomography (OCT) are the commonly used techniques.
If history and examination are normal but the uveitis is granulomatous, recurrent or bilateral, further investigations are necessary to look for underlying causes. The exact nature of the investigation is guided by clinical suspicion. Investigations might include:
FBC.
ESR.
Antinuclear antibody.
Angiotensin-converting enzyme.
Rapid plasma reagin.
HLA testing.
Mantoux test.
Quantiferon gold.
CXR (to exclude sarcoidosis or tuberculosis).
Urinalysis.
Infectious workup - eg, depending on presentation, toxoplasma, Lyme disease, HIV, syphilis.
Laboratory tests on a sample of aqueous or vitreous may be helpful where infection or malignancy is suspected.
Associated diseases3 4 16
48-70% of cases of uveitis are idiopathic.
About 50% of patients with acute anterior uveitis are HLA-B27-positive.
Some infections are specifically associated with anterior uveitis, including herpes simplex and herpes zoster.
Common associations are listed here: fuller lists can be obtained in the 'Further Reading and References' section, below.
Non-granulomatous disease
This is associated with:
Sacroiliitis
Seronegative arthropathies:
Early sarcoidosis
Early tuberculosis
Early syphilis
Drug-induced uveitis
Traumatic uveitis
Granulomatous anterior and posterior uveitis
These are associated with:
Herpes zoster virus
In AIDS:
Cytomegalovirus
Human syncytial virus
Cryptococcosis
Candidiasis
Non-infectious causes of posterior disease
These include:
Behçet's disease.
Sacroiliitis (usually bilateral).
Lymphoma.
VKH syndrome.
Lens-induced uveitis.
Posterior uveitis - may also be associated with autoimmune retinal vasculitis.
Management4 12
Refer people with suspected uveitis to an ophthalmologist within 24 hours. Do not initiate treatment for recurrent uveitis in primary care, unless asked to do so by an ophthalmologist: delay in appropriate management can lead to the development of significant complications and irreversible loss of vision
.
The aims of treatment are to control inflammation, prevent visual loss and minimise long-term complications of the disease and its treatment. There is no standard regimen: treatment is determined by the type of uveitis, whether it is secondary to infection and whether it is likely to threaten sight. Systemic drugs are reserved for sight-threatening posterior disease.
All patients with anterior disease and most with intermediate or posterior disease require treatment.
In chronic uveitis, treatment is usually indicated if the visual acuity has fallen to less than 6/12, or if the patient is experiencing visual difficulties.
Cycloplegic drugs
Cycloplegic-mydriatic drugs (eg, cyclopentolate 1%) are used to paralyse the ciliary body. This relieves pain and prevents adhesions between the iris and lens.
Steroids
Corticosteroids are used to reduce inflammation and prevent adhesions in the eye. They may be given topically, orally, intravenously, intramuscularly, or by periocular or intraocular injection, depending on the severity of the uveitis. Corticosteroids are reduced slowly, as withdrawing them too quickly may lead to rebound inflammation.
Many patients with unilateral chronic uveitis can be managed with topical corticosteroids, with periocular corticosteroids for macular oedema and visual loss.
Systemic corticosteroids are the mainstay of systemic treatment for patients with chronic uveitis: the usual indication for treatment is the presence of macular oedema and visual acuity of less than 6/12.
Immunosuppressors
These may be required for people with severe or chronic uveitis. These include methotrexate or mycophenolate, and tumour necrosis factor (TNF) inhibitors such as adalimumab.
Ciclosporin and tacrolimus are also useful in selected patients.
Adjunctive therapy17
Infectious uveitis (bacterial, viral, fungal, or parasitic) is treated with an appropriate antimicrobial drug as well as corticosteroids and cycloplegics4 . See also the separate Chorioretinal Inflammation article.
Severe or chronic uveitis may be treated with laser phototherapy, cryotherapy or vitrectomy.
Biologics, which target mediators of the inflammation cascade, may offer the potential to provide more effective and less toxic treatment18 .
Ongoing research is examining the use of immune modulators such as tumour necrosis factor alpha-blockers (eg, etanercept, infliximab) and interleukin-2 receptor blockers.
Interferon alfa may also have potential in treating refractory sight-threatening uveitis from a variety of causes.
Azathioprine has been found to be useful in steroid-resistant autoimmune uveitis.
The antivascular endothelial growth factor drugs (eg, ranibizumab, Lucentis®) which are currently used for 'wet' age-related macular degeneration may have a role in managing chronic, non-infective uveitis but are not yet licensed for this use4 . Intravitreal injection carries a small inherent risk of complications (including sight loss) in itself.
Research is also focused on less invasive sustained ocular drug delivery systems, including episcleral implants, nanospheres and cyclodextrin particles18 .
Considerations prior to initiating treatment
These include:
Supporting evidence for specific treatments is sparse.
IOP should be checked and herpes simplex virus keratitis ruled out before starting topical corticosteroids.
Steroid treatment should only be initiated in consultation with an ophthalmologist.
Surgery19
Surgery is considered in a small proportion of patients with severe or intractable disease or where a diagnostic vitreous sample is required (eg, to diagnose infection or malignancy).
Surgery may also be required for complications such as cataract, glaucoma and vitreoretinal problems; however, except in emergency situations, it should be contemplated only once the uveitis is controlled, ideally for at least three months.
Vitrectomy may be helpful when there is substantial opacity but also may improve disease control, particularly in younger patients .
Monitoring18
Monitoring is mainly by clinical examination but some investigations provide a useful adjunct:
OCT is helpful in looking for macular causes of worsening vision.
Fluorescein angiography is helpful in assessing retinal vascular involvement.
The only patient group currently screened for uveitis is children with juvenile idiopathic arthritis.
Monitoring for efficacy of treatments, adverse effects and development of complications usually takes place in secondary care, but GPs may be asked to monitor patients on long-term treatments such as corticosteroids and disease-modifying antirheumatic drugs (DMARDs)12 .
Complications
The main complication of uveitis is visual loss as a result of one or more of the following list (the first two being the leading causes)4 :
Cystoid macular oedema.
Secondary cataract.
Acute rise inIOP, with or without glaucoma, either as a direct consequence of the inflammatory process or secondary to steroid treatment:
Some patients, especially those with a history of glaucoma, are prone to developing high IOP when on steroid treatment and require co-treatment to reduce it. Treatment can be stopped when the steroid treatment is stopped if there is no pre-existing glaucoma.
Vitreous opacities (inflammatory debris or haemorrhage).
Neovascularisation of the retina, optic nerve, or iris.
Macular ischaemia, vascular occlusions and optic neuropathy - can be complications of posterior uveitis.
Posterior synechiae are a common complication of anterior uveitis; they can cause blockage of the aqueous flow, leading to a rise in IOP. They also complicate cataract surgery.
Prognosis
Anterior uveitis can be a self-limiting condition. The factors determining which cases will resolve without intervention are unclear.
With prompt and effective treatment, there is usually a good visual outcome (one study found that 91% of patients retain normal vision)4 .
Relapse after a first episode of acute anterior uveitis is very common, particularly in patients aged 18-35 years. Recurrence rates as high as 66% have been reported, although there is a wide range in the number of reported attacks and the time period between them, which can be many years20 21 .
Newer treatments may offer improved outcomes for previously refractory cases18 .
The prognosis of chronic granulomatous uveitis depends on the cause and on how promptly any underlying condition is recognised and treated.
Dr Mary Lowth is an author or the original author of this leaflet.
Further reading and references
- Agrawal RV, Murthy S, Sangwan V, et al; Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010 Jan-Feb;58(1):11-9. doi: 10.4103/0301-4738.58468.
- Deschenes J, Murray PI, Rao NA, et al; International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008 Jan-Feb;16(1):1-2.
- Duplechain A, Conrady CD, Patel BC, et al; Uveitis
- Guly CM, Forrester JV; Investigation and management of uveitis. BMJ. 2010 Oct 13;341:c4976. doi: 10.1136/bmj.c4976.
- Gutteridge IF, Hall AJ; Acute anterior uveitis in primary care. Clin Exp Optom. 2007 Mar;90(2):70-82.
- Harthan JS, Opitz DL, Fromstein SR, et al; Diagnosis and treatment of anterior uveitis: optometric management. Clin Optom (Auckl). 2016 Mar 31;8:23-35. doi: 10.2147/OPTO.S72079. eCollection 2016.
- McCluskey PJ, Towler HM, Lightman S; Management of chronic uveitis. BMJ. 2000 Feb 26;320(7234):555-8. doi: 10.1136/bmj.320.7234.555.
- Barisani-Asenbauer T, Maca SM, Mejdoubi L, et al; Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012 Aug 29;7:57. doi: 10.1186/1750-1172-7-57.
- Egwuagu CE, Sun L, Kim SH, et al; Ocular Inflammatory Diseases: Molecular Pathogenesis and Immunotherapy. Curr Mol Med. 2015;15(6):517-28.
- Jones NP; The Manchester Uveitis Clinic: the first 3000 patients--epidemiology and casemix. Ocul Immunol Inflamm. 2015 Apr;23(2):118-26. doi: 10.3109/09273948.2013.855799. Epub 2013 Dec 2.
- Rathinam SR, Babu M. Algorithmic approach in the diagnosis of uveitis. Indian Journal of Ophthalmology. 2013;61(6):255-262. doi:10.4103/0301-4738.114092.
- Uveitis; NICE CKS, November 2019 (UK access only)
- Babu BM, Rathinam SR; Intermediate uveitis. Indian J Ophthalmol. 2010 Jan-Feb;58(1):21-7. doi: 10.4103/0301-4738.58469.
- Davis JL, Madow B, Cornett J, et al; Scale for photographic grading of vitreous haze in uveitis. Am J Ophthalmol. 2010 Nov;150(5):637-641.e1. doi: 10.1016/j.ajo.2010.05.036. Epub 2010 Aug 16.
- Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005 Sep;140(3):509-16.
- Martin TM, Rosenbaum JT; An update on the genetics of HLA B27-associated acute anterior uveitis. Ocul Immunol Inflamm. 2011 Apr;19(2):108-14. doi: 10.3109/09273948.2011.559302.
- Koronis S, Stavrakas P, Balidis M, et al; Update in treatment of uveitic macular edema. Drug Des Devel Ther. 2019 Feb 19;13:667-680. doi: 10.2147/DDDT.S166092. eCollection 2019.
- Ratay ML, Bellotti E, Gottardi R, et al; Modern Therapeutic Approaches for Noninfectious Ocular Diseases Involving Inflammation. Adv Healthc Mater. 2017 Dec;6(23). doi: 10.1002/adhm.201700733. Epub 2017 Oct 16.
- Murthy SI, Pappuru RR, Latha KM, et al; Surgical management in patient with uveitis. Indian J Ophthalmol. 2013 Jun;61(6):284-90. doi: 10.4103/0301-4738.114103.
- Grunwald L, Newcomb CW, Daniel E, et al; Risk of Relapse in Primary Acute Anterior Uveitis. Ophthalmology. 2011 Jun 14.
- Natkunarajah M, Kaptoge S, Edelsten C. Risks of relapse in patients with acute anterior uveitis. The British Journal of Ophthalmology. 2007;91(3):330-334. doi:10.1136/bjo.2005.083725.
Article History
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 18 Feb 2026
19 Feb 2021 | Latest version

Feeling unwell?
Assess your symptoms online for free