Non-diabetic retinal vascular disease
Peer reviewed by Dr Pippa Vincent, MRCGPLast updated by Dr Colin Tidy, MRCGPLast updated 21 Apr 2023
Meets Patient’s editorial guidelines
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find one of our health articles more useful.
In this article:
Continue reading below
Overview
This article provides an overview of vasculopathies affecting the retina. See also separate Diabetic Retinopathy and Diabetic Eye Problems, The Eye in Systemic Disease and Retinopathy of Prematurity articles.
Retinal anatomy1
The retina is a many layered structure. From the inner (vitreous) surface to the choroid these are the internal limiting membrane, nerve fibre layer, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, outer nuclear layer, external limiting membrane, rod and cone inner and outer segments and a single layer of cells called the retinal pigment epithelium (RPE).
The only cells which are sensitive to light are the photoreceptor cells, comprising the rods and cones (for vision) and the photosensitive ganglion cells for entrainment and reflex responses to light. Neural signals from the rods and cones are processed by other nerve cells in the retina.
The macula has a high density of cones, ganglion cells, and pigment. The central 1.5 mm area of the macula is the fovea. Within the fovea is a roughly circular avascular area, the foveal avascular zone, which contains only cones. These layers vary in thickness around the retina, becoming thinner towards the fovea and disappearing altogether at the fovea centralis, leaving a small area of uncovered photoreceptors.
Retinal anatomy
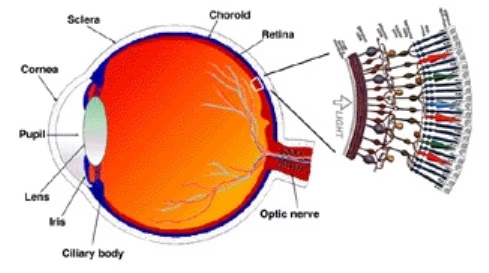
د.مصطفى الجزار, CC BY-SA 3.0, via Wikimedia Commons
Retinal blood supply
The ophthalmic artery comes directly off the internal carotid artery and runs through the optic canal. Its first branch is the central retinal artery which penetrates, then runs in the centre of, the optic nerve, to enter the eye where it divides and supplies the inner neural retina.
The ophthalmic artery then gives rise to the lacrimal artery, followed by a number of posterior ciliary arteries. These perforate the sclera near the optic nerve and are end arteries (they do not anastomose with any other artery. They supply the posterior uveal tract including the outer neural retina and the choroid.
Thus, the retina is has two blood supplies:
The outer RPE, the photoreceptors and a few of the overlying tissue layers are supplied by the choroid, itself supplied by the posterior ciliary arteries. There is a blood-retina barrier between the RPE and the photoreceptors, which protects it from large toxic molecules.
The inner neural retina is supplied by the central retinal artery. The central retinal artery divides into two equal superior and inferior branches nasally and temporally which supply all the inner layers of the neural retina, with corresponding venous drainage.
Clinical significance of the two blood supplies
Depending on what type of vascular problem arises and where exactly it occurs, different layers of the retina wil be affected and others spared. For example, an embolus in the ophthalmic artery will affect the blood supply to all layers of the entire retina, whereas a smaller embolus in the inferior nasal branch of the artery will only affect the inner neural retina in that area, sparing the photoreceptors and limiting any symptoms.
Retinal imaging2
Although fundoscopy remains a useful tool, digital fundus cameras produce high-resolution digital images which are easily acquired. Computer-assisted image analysis can then be used to characterise more accurately abnormalities of the retinal microvasculature. They also provide more permanent records of progression of retinal abnormalities as diseases progress and in response to therapy.
Retinal artery occlusions
See the separate Retinal Artery Occlusions article.
Continue reading below
Retinal vein occlusions
See the separate Retinal Vein Occlusions article.
Problems related to systemic disease
Hypertensive retinopathy2 3
Description
This is a spectrum of microvascular abnormalities associated with persistently raised blood pressure. Initially, there is arterial narrowing (visible as copper wiring). This is followed by vascular leakage and subsequent arteriosclerosis, which is graded 1-4. It has a characteristic appearance on fundoscopy (described below). Rarely, choroidal changes may occur, usually in the context of an acute hypertensive crisis (accelerated hypertension) in a young adult.3
Hypertensive retinopathy symptoms (presentation)4
Chronic hypertension (blood pressure > 140/90 mm Hg): usually asymptomatic although patients may have slightly decreased vision. Fundoscopy reveals bilateral attenuation of arterial vessels ('copper or silver wiring'), arteriovenous nipping (where the arteries cross the veins) and, eventually, haemorrhage and exudates.
Malignant (accelerated) hypertension (clinic blood pressure >180/110 mm Hg): patients may have headaches and decreased vision. On fundoscopy, you may see hard exudates appear as a 'macular star' (thin white streaks radiating around the macula), disc swelling, cotton wool spots, flame haemorrhages and arterial or venous occlusions.
Hypertensive retinopathy treatment and management
Management should be aimed at controlling the hypertension. Accelerated hypertension is a medical emergency.
Outcome
Hypertensive retinopathy is associated with a two- to three-fold increase in the risk of stroke. These increases appear independent of the risks associated with raised blood pressure.2
Patients with choroidal changes are at risk of developing retinal vein occlusions described above, particularly when there are concurrent risk factors (such as smoking and hyperlipidaemia).3
Ocular ischaemic syndrome5
Description
This arises as a result of chronic ocular hypoperfusion secondary to severe atherosclerotic carotid stenosis. It is relatively uncommon.
Ocular ischaemic syndrome symptoms (presentation)
It usually presents in the seventh decade of life (range 50-80 years), often in association with diabetes, hypertension, coronary heart disease or cerebrovascular disease. It may also occur in the context of giant cell arteritis.
Male:female ratio is 2:1.
Symptoms are unilateral and include a gradually decreasing vision (over weeks or months; although (rarely) it can be sudden), periorbital pain and prolonged recovery of images after exposure to bright light. There may be a history of amaurosis fugax.
Signs include a red eye, corneal oedema, a mid-dilated poorly reacting pupil and there may be rubeosis iridis. Fundoscopy shows venous dilatation, micro-aneurysms, neovascularisation and disc oedema.
Ocular ischaemic syndrome treatment and management
Urgent referral to ophthalmology: management involves a multidisciplinary team, including the cardiologist, neurologist and vascular and neurological surgeons. Patients will be treated with topical steroids and long-acting cycloplegic agents, with laser treatment to any new vessel growth. Any associated raised intra-ocular pressure will also be treated. The underlying carotid disease needs addressing; carotid stenosis > 60% needs vascular surgical review.
Outcome: both visual recovery and systemic outcome are poor. There is a ~40% five-year mortality rate, usually from cardiac disease.5
Sickle cell retinopathy6 7
Description
The most severe forms of retinopathy are associated with sickle cell C disease and sickle cell thalassaemia; however, all types of sickle cell disease can give rise to retinopathy. The impacted sickle cells occlude arteries (stage 1), giving rise to peripheral arteriovenous anastomoses (stage 2) and then sprouting of new vessels from these anastomoses (stage 3). Trivial ocular trauma may precipitate vitreous haemorrhage (stage 4) resulting in subsequent fibrovascular proliferation and tractional retinal detachment (stage 5).
Sickle cell retinopathy symptoms (presentation)
There are usually no intra-ocular symptoms until stage 4 when there may be floaters and stage 5, which presents with flashes and floaters ± loss of vision.
Signs will depend on the stage of the disease. There may be comma-shaped vessels, particularly in the inferior conjunctiva and there may be iris atrophy.
Haemoglobinopathies can also give rise to non-proliferative retinopathies characterised by arteriosclerosis, venous tortuosity, equatorial 'salmon patches' (superficial retinal haemorrhages), 'black sunbursts' (intraretinal haemorrhages), macular oedema, micro-aneurysms and cotton wool spots.
Sickle cell retinopathy treatment and management
This is directed towards screening, early detection, prevention and early treatment of retinopathy, in order to avoid vitreous haemorrhage. Patients at stages 3-5 may benefit from laser treatment to abnormal vessels and surgery to address tractional fibrosis and detachment.
Outcome
This depends on the stage and whether neovascularisation can be controlled - which ultimately depends on the underlying systemic illness.
Purtscher's retinopathy8
Description
A rare retinopathy resulting from microvascular damage and occlusion as a result of severe head trauma, chest compression injury or other crush injuries involving broken bones or fat emboli. It can be caused by a number of systemic diseases (eg, pancreatic disease, connective tissue diseases, lymphomas and thrombocytic thrombocytopenic purpura and following bone marrow transplantation). It can occur during pregnancy or delivery. The estimated annual incidence is 0.24 cases per million population per year in the UK.
Purtscher's retinopathy symptoms (presentation)
Presents with sudden, severe visual loss which is usually bilateral.
Fundoscopy reveals multiple white retinal patches and haemorrhages around the disc.
Purtscher's retinopathy treatment and management
There is no established treatment for the eyes specifically, although isolated case reports have suggested possible benefits from systemic steroids or hyperbaric oxygen. Management of the underlying cause is the mainstay of treatment.
Outcome
Fundal changes often resolve within a few weeks and spontaneous visual recovery of at least 2 Snellen lines is seen in half of the cases.
Non-infectious retinal microvascular retinopathy ('HIV retinopathy')9
Description
HIV patients can experience a variety of ocular complications, many of these infectious in nature.
Before the introduction of highly active antiretroviral therapy (HAART) 50-70% of people living with HIV (PLHIV) developed retinopathy. This number has reduced considerably since the introduction of HAART.
Non-infectious retinal microvascular retinopathy symptoms (presentation)
HIV retinopathy tends to be asymptomatic but 3% of patients experience severe visual loss.
Fundoscopy reveals a diabetic type of appearance (cotton wool spots and micro-aneurysms) ± ischaemic maculopathy.
Non-infectious retinal microvascular retinopathy treatment and management
There is no specific treatment. Digital fundal imaging has demonstrated that narrowing of arterioles is associated with the duration of treatment with HAART, or with the prolonged inflammatory state associated with AIDS.
Outcome
The severity of HIV retinopathy is correlated with worsening visual morbidity (eg, visual field loss). It may also serve as a marker for the severity of systemic atherogenic vascular disease which is also associated with chronic HIV infection and prolonged treatment with HAART.10 11
Continue reading below
Other retinal vascular problems3
Retinal artery macro-aneurysm
Description
This localised dilatation of a retinal arteriole results from idiopathic weakening of the vessel wall, which leads to focal outpouching and aneurysm formation).
It tends to occur in older (60-80 years), hypertensive women.3
It differs from the micro-aneurysms of diabetic retinopathy in that macro-aneurysms are larger in size and tend to occur singly.
Retinal artery macro-aneurysm symptoms (presentation)
The macroaneurysms are usually unilateral (90% of cases).
They may be an incidental finding or may present with sudden painless visual loss from haemorrhage.
More commonly there is gradual visual impairment due to insidious macular oedema and hard exudate formation (seen around the aneurysm on fundoscopy).
Retinal artery macro-aneurysm treatment and management
Where aneurysm rupture leads to haemorrhage management is restricted to observation until it involutes (as laser treatment cannot be carried out through the haemorrhage). Laser treatment is then performed to the lesion. The use of intravitreal injection of vascular endothelial growth factor (VEGF) inhibitors, such as ranibizumab or bevacizumab, have been used with good outcomes in a few isolated cases.12
Outcome
Involution of the haemorrhage is very common but, occasionally, there is a chronic leak resulting in permanent loss of central vision.
Primary retinal telangiectasia
Description
This group of rare, idiopathic disorders may be congenital or acquired. There are various vascular abnormalities (tortuosities, leakages, aneurysms and deposition of hard exudates) which often progress over time.
Primary retinal telangiectasia symptoms (presentation)
The condition may be asymptomatic until later in life when progression has occurred and presentation is then with painless blurring of vision.
The condition is usually unilateral.
Coats' disease is the most severe of this group of disorders and often presents early (typically affecting boys, presenting in the first decade of life, often by 5 years of age). There may be a retinal detachment associated with this form of disease (often seen as leukocoria).13
Primary retinal telangiectasia treatment and management
This depends on the subtype and severity. Management ranges from observation to photocoagulation laser therapy, cryotherapy, vitreoretinal surgery and intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF).14
Outcome
This is generally poor.
Radiation retinopathy15
Description
Radiation retinopathy may develop following treatment of intra-ocular tumours with radiotherapy. It can also occur following treatment of sinus, orbital or nasopharyngeal tumours.
The risk of retinopathy increases with radiation dose.
Radiation retinopathy symptoms (presentation)
Patients usually present six months to three years post-therapy with a painless gradual loss of vision.
Fundoscopy shows micro-aneurysms, macular oedema, hard exudates and flame-shaped retinal haemorrhages. There may be associated disc oedema ± proliferative retinopathy and tractional retinal detachment.
A variant is the acute response to high-dose radiation: the retina shows evidence of necrosis with widespread vascular occlusion, cotton wool spots and widespread haemorrhages.
Radiation retinopathy treatment and management
This depends on the exact findings but this may include topical steroids, anti-VEGF, laser treatment or surgery.
Outcome
This depends on the degree of retinopathy. Poor prognostic features include optic nerve involvement and neovascularisation.
Dr Mary Lowth is an author or the original author of this leaflet.
Further reading and references
- J. Reynolds and S. Olitsky (eds.), Pediatric Retina, 39 DOI: 10.1007/978-3-642-12041-1_2, © Springer-Verlag Berlin Heidelberg 2011
- Henderson AD, Bruce BB, Newman NJ, et al; Hypertension-related eye abnormalities and the risk of stroke. Rev Neurol Dis. 2011;8(1-2):1-9.
- Colucciello M; Retinal vascular disease in hypertension. Risk factor modification optimizes vision outcomes. Postgrad Med. 2005 Jun;117(6):33-8, 41-2.
- Hypertension in adults: diagnosis and management; NICE (August 2019 - last updated November 2023)
- Mendrinos E, Machinis TG, Pournaras CJ; Ocular ischemic syndrome. Surv Ophthalmol. 2010 Jan-Feb;55(1):2-34. doi: 10.1016/j.survophthal.2009.02.024. Epub .
- Fadugbagbe AO, Gurgel RQ, Mendonca CQ, et al; Ocular manifestations of sickle cell disease. Ann Trop Paediatr. 2010;30(1):19-26. doi: 10.1179/146532810X12637745451870.
- Ribeiro MVMR, Juca JVO, Alves ALCDS, et al; Sickle cell retinopathy: A literature review. Rev Assoc Med Bras (1992). 2017 Dec;63(12):1100-1103. doi: 10.1590/1806-9282.63.12.1100.
- Agrawal A, McKibbin M; Purtscher's retinopathy: epidemiology, clinical features and outcome. Br J Ophthalmol. 2007 Nov;91(11):1456-9. Epub 2007 Jun 7.
- Pathai S, Weiss HA, Lawn SD, et al; Retinal Arterioles Narrow with Increasing Duration of Anti-Retroviral Therapy in HIV Infection: A Novel Estimator of Vascular Risk in HIV? PLoS One. 2012;7(12):e51405. doi: 10.1371/journal.pone.0051405. Epub 2012 Dec 10.
- Gangaputra S, Kalyani PS, Fawzi AA, et al; Retinal vessel caliber among people with acquired immunodeficiency syndrome: relationships with disease-associated factors and mortality. Am J Ophthalmol. 2012 Mar;153(3):434-444.e1. doi: 10.1016/j.ajo.2011.08.028. Epub 2011 Oct 22.
- Kalyani PS, Fawzi AA, Gangaputra S, et al; Retinal vessel caliber among people with acquired immunodeficiency syndrome: relationships with visual function. Am J Ophthalmol. 2012 Mar;153(3):428-433.e1. doi: 10.1016/j.ajo.2011.08.027. Epub 2011 Oct 22.
- Golan S, Goldenberg D, Goldstein M; Long-term follow-up of intravitreal bevacizumab in retinal arterial macroaneurysm: a case report. Case Report Ophthalmol. 2011 Sep;2(3):387-91. doi: 10.1159/000334788. Epub 2011 Dec 13.
- Sen M, Shields CL, Honavar SG, et al; Coats disease: An overview of classification, management and outcomes. Indian J Ophthalmol. 2019 Jun;67(6):763-771. doi: 10.4103/ijo.IJO_841_19.
- Knutsson KA, De Benedetto U, Querques G, et al; Primitive retinal vascular abnormalities: tumors and telangiectasias. Ophthalmologica. 2012;228(2):67-77. Epub 2012 Jun 23.
- Gupta A, Dhawahir-Scala F, Smith A, et al; Radiation retinopathy: case report and review. BMC Ophthalmol. 2007 Apr 5;7:6. doi: 10.1186/1471-2415-7-6.
Article History
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 19 Apr 2028
21 Apr 2023 | Latest version

Feeling unwell?
Assess your symptoms online for free