Vitreous haemorrhage
Peer reviewed by Dr Hayley Willacy, FRCGP Last updated by Dr Colin Tidy, MRCGPLast updated 10 May 2023
Meets Patient’s editorial guidelines
- DownloadDownload
- Share
- Language
- Discussion
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find the Vitreous haemorrhage article more useful, or one of our other health articles.
In this article:
What is vitreous haemorrhage?1 2
Vitreous haemorrhage, or bleeding into the vitreous humour, is one of the most common causes of sudden painless visual loss. The degree of visual loss varies from haziness and floaters to complete obscuration of vision.
Blood may get into the vitreous through disruption of normal retinal vessels, bleeding from diseased retinal vessels or abnormal new vessels, and by extension through the retina from other sources.
Haemorrhage in the vitreous results in clot formation. Once bleeding stops it is followed by slow clearance of blood (1% per day). The inflammatory response to blood in the vitreous is relatively muted, which helps reduce the chance of permanent loss of clarity.
Whilst diagnosis of vitreous haemorrhage is relatively straightforward, determining the underlying cause may be more difficult because visualisation of the posterior structures of the eye may be obscured by blood.
Anatomy of the vitreous
Back to contentsSide view of the structure of the eye
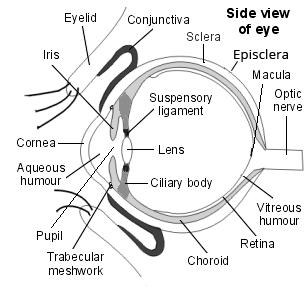
The vitreous is gelatinous and avascular. Healthy vitreous is relatively inelastic and impervious to cells and debris. It helps maintain the transparency and structure of the eye. The volume of the vitreous body of an adult is around 4 ml and it forms 80% of the globe of the eye. 99% of this is water and the remaining 1% is mainly collagen and hyaluronic acid.
The outer limits of the vitreous are formed by condensations of fibrils that form attachments at the periphery of the retina, posterior ciliary body and posterior lens capsule and around the optic nerve.
Continue reading below
How common is vitreous haemorrhage? (Epidemiology)
Back to contentsVitreous haemorrhage is a relatively common cause of visual loss. Incidence by ethnicity, sex and age corresponds to the incidence of the underlying causes.
Pathophysiology1 3 4
Back to contentsSystemic vascular conditions such as diabetes, hypertension, vascular occlusions, posterior vitreous detachment, and retinal tears are common causes of VH in middle-aged and older patients.
Exudative haemorrhagic chorioretinopathy may result in VH in patients older than 75 years.
Ocular trauma, inflammatory conditions/vasculitides, or haematological conditions such as haemoglobinopathies, and sickle cell disease, are important causes of VH in the young.
VH in childhood may be seen in patients with retinopathy of prematurity, persistent hyperplastic primary vitreous, Coats' disease, and familial exudative vitreoretinopathy.
Overall, the most common underlying causes of vitreous haemorrhage are:
Proliferative diabetic retinopathy. This follows retinal ischaemia via factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and insulin-like growth factor 1 (IGF-1). The new vessels grow into the vitreous, are fragile and bleed easily. Over half of all cases relate to diabetic retinopathy.
Posterior vitreous detachment (PVD). This is covered in detail in the separate Posterior Vitreous Detachment (PVD) article. It is associated with a retinal tear or break in 70-95% of cases.
Ocular trauma. The most common cause in children and young adults:
Open globe injury may cause bleeding in all layers of the eye, including vitreous haemorrhage.
Closed globe injury from blunt trauma. Acute antero-posterior compression of the globe causes bulging of the eye in a coronal plane. Younger patients have a more formed vitreous which is strongly adherent to the retina. The strongest attachment is near the equator and it therefore exerts strong traction on the retina. This can lead to tears and vessel rupture, or to retinal dialysis (a separation of the peripheral retina from the ora serrata or just posterior to it, which appears as a semi-circular-shaped break).
Shaking injuries (shaken baby syndrome) may cause haemorrhage in all ocular layers, including vitreous haemorrhage.
Together these account for up to 90% of cases. Other less common causes include:
Retinal macroaneurysm rupture. Retinal macroaneurysms are usually associated with systemic hypertension and atherosclerosis. They are associated with macular exudation or haemorrhage and are most common in the sixth to seventh decade.
Proliferative retinopathy after retinal vein occlusion.
Proliferative sickle cell retinopathy (and other haemoglobinopathies).
Subarachnoid haemorrhage (Terson's syndrome): vitreous haemorrhage is seen in 10-40% of patients with subarachnoid haemorrhage. It is not a direct extension of subarachnoid hemorrhage into the eye via the optic nerve sheath. Instead, increased intracranial pressure causes increased pressure in retinal venules, leading to rupture.
Neovascular age-related macular degeneration (AMD): choroidal neovascularisation due to AMD can cause 'breakthrough' bleeding into the vitreous.
Ocular tumour: tumours of the choroid may develop abnormal blood vessels and bleed.
Retinopathy of prematurity promotes neovascularisation.
Risk factors
Risk factors for neovascularisation (eg, diabetes).
Trauma.
Anticoagulants and antiplatelet agents do not cause vitreous haemorrhage but they may enhance bleeding from pathology.5
Patients with disorders of coagulation might be expected to have an increased risk of spontaneous vitreous haemorrhage but cases are rare.
Individuals with high myopia have an increased risk of retinal tears, retinal detachment and associated vitreous haemorrhage.
Continue reading below
Vitreous haemorrhage symptoms (presentation)1
Back to contentsPresentation is usually with sudden, painless visual loss or haze. Patients may also describe:
A red hue.
New-onset floaters and 'cobwebs'.
Symptoms which may be worse in the morning if blood settles on the macula during sleep.
A history of diabetes, hypertension, sickle cell disease, ocular surgery or trauma.
Variable visual acuity, depending on the size of the haemorrhage. It may be dramatically reduced.
Haze which may appear greenish following haemoglobin breaking down In chronic vitreous haemorrhage.
Investigation1
Back to contentsBlood in the vitreous is easily detected. A view through to the retina may be, although is not always, possible as dispersed vitreous haemorrhage may totally obscure the back of the eye. Examination of suspected vitreous haemorrhage should always include:
Intraocular pressures.
Best corrected visual acuity.
Dilated fundoscopy may reveal haemorrhage spread through the vitreous, or the bleed may conform in shape to the underlying structures.
Slit-lamp examination reveals red blood cells in the anterior vitreous.
Gonioscopy to look for new vessels in the drainage angle.
If there is acute PVD, retinal tear or detachment must be ruled out using scleral depression.
The other eye must also be examined.
Ultrasonography can be used to detect the blood, PVD, retinal tears, retinal detachment, tractional membranes, intraocular tumours and foreign bodies. This is particularly helpful if the view of the retina is obscured.
Fluorescein angiography may help neovascularisation.
Orbital CT is indicated in cases of open globe injury, to allow assessment of the integrity of other structures in the orbit and to rule our intraocular foreign body.
Blood pressure should also be checked.
Differential diagnosis
Back to contentsThe differential diagnosis contains all causes of bleeding into the vitreous. This includes:
Causes of neovascularisation
Diabetes.
Hypertensive retinopathy.
Sickle retinopathy.
Radiation retinopathy.
Familial exudative vitreoretinopathy.
Retinal vasculitis (eg, sarcoid).
AMD.
Other causes of retinal bleeding
Macroaneurysm.
Familial retinal arteriolar tortuosity.
Pathological myopia.
Infectious chorioretinopathy - eg, histoplasmosis.
Choroidal tumours, melanoma, retinoblastoma.
Trauma.
Open or closed globe injury.
Blood disorders - eg, leukaemia, thrombocytopenia.
Terson's syndrome.
Ocular inflammatory conditions - eg, panuveitis.
Primary CNS lymphoma.
Eales' disease (an idiopathic vasculopathy that usually affects young aduts).
Syphilis can mimic almost any intraocular inflammatory condition.
Vitreous haemorrhage treatment and management1 3 6
Back to contentsSudden visual loss needs emergency referral to an ophthalmologist. Management varies with the underlying cause, which needs to be treated as soon as possible.
Treatment options include observation, laser photo-coagulation, cryotherapy, intravitreal injections of anti-vascular endothelial growth factor, and surgery. Pars plana vitrectomy remains the cornerstone of management.
Observation
Fresh vitreous haemorrhage often clears in days to weeks to allow evaluation of retina. However, retinal detachment must be excluded urgently.
In vitreous haemorrhage of unknown aetiology and without retinal detachment (on ultrasonography), the patient should rest with the head elevated. Revaluate after 3-7 days to ascertain the possible source of haemorrhage.
No topical or systemic medication is needed in this situation as none is of proven benefit. Oral ascorbic acid (vitamin C) is sometimes given to speed clearance (although not clinically proven), as there is more liquefaction and loss of gel structure in eyes with exogenous ascorbic acid.
In patients with known aetiology and with attached retina, re-evaluation is done after 3-4 weeks. This includes post-laser or post-vitrectomy recurrent vitreous haemorrhages, vitreous haemorrhage in Tersons' syndrome or after acute PVD and haemorrhage associated with bleeding diathesis.
In eyes with attached macula, waiting 2-3 weeks for PVD to occur increases the technical ease and outcomes of surgery. This includes eyes with penetrating trauma without retained intra-ocular foreign body (and without infection), fresh retinal detachment with vitreous haemorrhage and no PVD, Eales' disease without PVD and vitreous haemorrhage in closed globe injury without retinal detachment.
However, early surgery is generally recommended in vitreous haemorrhage associated with retinal detachment.
Laser photocoagulation
Laser photocoagulation in proliferative vasculopathies should start as soon as any part of the retina is visible.
In some eyes, this can begin using an indirect ophthalmoscope delivery system, later completed with slit-lamp delivery.
Rarely, transconjunctival laser can also be used for panretinal photocoagulation or treatment of retinal breaks if media haze due to vitreous haemorrhage, cataract, corneal oedema or poorly dilating pupil disallows adequate focusing of the transpupillary laser beam.
Panretinal photocoagulation will cause regression of neovascularisation and help reduce the risk of further haemorrhage.
Retinal breaks are treated with cryotherapy or laser photocoagulation.
Anterior retinal cryotherapy (ARC)
ARC has been used in eyes with fresh vitreous haemorrhage. It appears that ARC causes breakdown of blood-retinal barrier, which in turn leads to the clearance of liquefied blood.
Cryotherapy is more inflammatory than laser photocoagulation; it may promote formation of pre-retinal fibrin and can result in tractional retinal detachment. It should not be used in eyes that have not undergone previous laser, in eyes with tractional membranes and in eyes with vitreous haemorrhage of unknown aetiology. Careful ultrasonography is mandatory before ARC.
Vitrectomy
Early vitrectomy is indicated when the underlying pathology is likely to progress fast if untreated.
Eyes with attached retina, good PVD and non-clearing vitreous haemorrhage over 2-3 months.
Eyes with advanced proliferative retinopathy where the vitreous haemorrhage does not clear in 6-8 weeks after adequate laser therapy.
Vitreous haemorrhage in retinal detachment especially when associated with large retinal tears or open globe injury and vitreous haemorrhage due to AMD and idiopathic polypoidal choroidal vasculopathy (IPCV).
Vitrectomy can be delayed in eyes with well lasered proliferative retinopathy and attached retina where the recurrent haemorrhage is not secondary to active proliferation
Vitrectomy can be deferred until good PVD occurs in eyes with Tersons' syndrome, closed globe-injuries, post-cataract surgery vitreous haemorrhage, and vitreous haemorrhage in bleeding diathesis.
If the retina can be adequately visualised but safe treatment isn't possible.
If the retina cannot be adequately visualised and the aetiology is unclear.7
Neovascularisation of the iris or angle in the setting of new dense vitreous haemorrhage would also prompt earlier surgical intervention.
After blunt trauma, when there has been no improvement in 2-3 weeks, vitreous surgery can be helpful.8
Intravitreal anti-VEGF agents9
These agents (eg, bevacizumab) are used to cause regression of neovascularisation in proliferative retinopathies, particularly if there is no view allowing photocoagulation. However, there is anecdotal evidence that anti-VEGF injection may worsen tractional retinal detachment.
Many surgeons use pre-operative anti-VEGF agents before pars plana vitrectomy for vitreous haemorrhage in those with diabetes, as regression of neovascular membranes reduces intraoperative and postoperative bleeding.
Intravitreal injection of an anti-VEGF agent is usually indicated when the cause of vitreous haemorrhage is neovascular AMD.
Some studies suggest that intravitreal anti-VEGF agents produce partial or complete resolution of recent-onset haemorrhages in patients with proliferative diabetic retinopathy but evidence is inconclusive. Vitrectomy remains the treatment of choice where the bleed is more than three months old.
A Cochrane review in 2015 suggested that anti-VEGF lowers the incidence of early postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy.10
New strategies for the treatment of vitreous haemorrhage, such as pharmacological vitreous liquefaction, may be important in the future.
Advice for patients
Avoid strenuous activity, as an increase in blood pressure may disrupt a clot and cause new active bleeding.
Elevate the head of the bed to allow the blood to settle, improving their vision and assisting fundoscopic examination.
Bilateral patching and bedrest may facilitate settling of blood. However, patches must be removed immediately before examination or treatment, as normal eye movements quickly disperse the haemorrhage again, so this is difficult for patients, with often minimal gain.
Aspirin and other forms of anticoagulation do not worsen the condition and can be continued after a vitreous haemorrhage.
Complications1 3
Back to contentsThe following may be seen if blood remains in the vitreous for prolonged periods - a year or more:
Haemosiderosis bulbi is a serious but uncommon complication thought to be caused by iron toxicity as haemoglobin is broken down.
Proliferative vitreoretinopathy. After vitreous haemorrhage, fibrovascular proliferation can lead to scarring and subsequent retinal detachment.
Ghost cell glaucoma. Ghost cells are spherical, rigid, khaki-coloured red blood cells filled with denatured haemoglobin. They are seen only in long-standing vitreous haemorrhage. Their shape and rigidity can block the trabecular meshwork, resulting in ghost cell glaucoma.
Haemolytic glaucoma: free haemoglobin, haemoglobin-laden macrophages and red blood cell debris can block the trabecular meshwork.
Prognosis1 4 6
Back to contentsThis depends on the underlying cause. It is generally better in eyes without underlying disease.
Blood clears from the vitreous at around 1% per day. Blood outside the formed vitreous clears more quickly.
Blood also clears more quickly if the patient is younger, and after vitrectomy.
The prognosis is worst for patients with diabetes or AMD.
Where vitreous haemorrhage due to diabetic vitreous haemorrhage leads to vision of 5/200 or less, most patients do not clear spontaneously even after one year.
The prognosis in children is dependent on the aetiology: a substantial proportion has significant visual loss. Of bilateral cases, over half relate to shaken baby syndrome. Visual outcomes were poorest with penetrating trauma and best with regressed retinopathy of prematurity.
Dr Mary Lowth is an author or the original author of this leaflet.
Further reading and references
- Shaikh N, Srishti R, Khanum A, et al; Vitreous hemorrhage - Causes, diagnosis, and management. Indian J Ophthalmol. 2023 Jan;71(1):28-38. doi: 10.4103/ijo.IJO_928_22.
- Naik AU, Rishi E, Rishi P; Pediatric vitreous hemorrhage: A narrative review. Indian J Ophthalmol. 2019 Jun;67(6):732-739. doi: 10.4103/ijo.IJO_688_18.
- Goff MJ, McDonald HR, Johnson RN, et al; Causes and treatment of vitreous hemorrhage. Compr Ophthalmol Update. 2006 May-Jun;7(3):97-111.
- Spirn MJ, Lynn MJ, Hubbard GB 3rd; Vitreous hemorrhage in children. Ophthalmology. 2006 May;113(5):848-52.
- Kiernan DF, Hariprasad SM, Rusu IM, et al; Epidemiology of the association between anticoagulants and intraocular hemorrhage in patients with neovascular age-related macular degeneration. Retina. 2010 Nov-Dec;30(10):1573-8. doi: 10.1097/IAE.0b013e3181e2266d.
- El Annan J, Carvounis PE; Current management of vitreous hemorrhage due to proliferative diabetic retinopathy. Int Ophthalmol Clin. 2014 Spring;54(2):141-53. doi: 10.1097/IIO.0000000000000027.
- Dhingra N, Pearce I, Wong D; Early vitrectomy for fundus-obscuring dense vitreous haemorrhage from presumptive retinal tears. Graefes Arch Clin Exp Ophthalmol. 2007 Feb;245(2):301-4.
- Guo XR, Guo HY, Li YS, et al; The surgical timing and effects for vitreous hemorrhage caused by ocular blunt trauma. Zhonghua Yan Ke Za Zhi. 2003 Jul;39(7):419-21.
- Bhavsar AR et al; Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013 Mar;131(3):283-93. doi: 10.1001/jamaophthalmol.2013.2015.
- Smith JM, Steel DH; Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2015 Aug 7;8:CD008214. doi: 10.1002/14651858.CD008214.pub3.
Continue reading below
Article history
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 8 May 2028
10 May 2023 | Latest version

Ask, share, connect.
Browse discussions, ask questions, and share experiences across hundreds of health topics.

Feeling unwell?
Assess your symptoms online for free