Gastro-oesophageal reflux disease
Peer reviewed by Dr Doug McKechnie, MRCGPLast updated by Dr Colin Tidy, MRCGPLast updated 16 Aug 2024
Meets Patient’s editorial guidelines
- DownloadDownload
- Share
- Language
- Discussion
- Audio Version
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find the Acid reflux and oesophagitis article more useful, or one of our other health articles.
In this article:
Synonyms: gastro-esophageal reflux disease; reflux oesophagitis
See also the separate Childhood gastro-oesophageal reflux and Dyspepsia articles.
Continue reading below
What is gastro-oesophageal reflux disease?1
A certain amount of gastro-oesophageal reflux of acid is normal and there is a natural protective mechanism of the lower oesophagus. Gastro-oesophageal reflux disease (GORD) describes prolonged or excessive reflux that may cause breakdown of this protection with inflammation of the oesophagus (oesophagitis).
Gastro-oesophageal reflux disease is usually a chronic condition where there is reflux of gastric contents (particularly acid, bile, and pepsin) back into the oesophagus, causing predominant symptoms of heartburn and acid regurgitation. Atypical symptoms affecting the oropharynx and/or respiratory tract may occur, such as hoarseness, cough, asthma, and dental erosions.
Laryngopharyngeal reflux2
Laryngopharyngeal reflux is caused by reflux of gastric contents into the pharynx or larynx, which leads to symptoms of throat clearing, hoarseness, pain, globus sensation, cough, excess mucus production in the throat, and dysphonia.
Functional laryngeal disorders and laryngeal hypersensitivity can present as laryngopharyngeal reflux symptoms with or without gastro-oesophageal reflux disease.
One large study found that proton pump inhibitors do not benefit patients with persistent throat symptoms.3 It has been suggested that medicines such as Gaviscon® may be more effective.
How common is GORD? (Epidemiology)
Back to contentsIt is estimated that GORD affects 10-30% of the adult population in developed countries. The prevalence of GORD increases with age, and it is slightly more common in women.1
There is a spectrum of disorders ranging from the most common endoscopy-negative gastro-oesophageal reflux disease (GORD) to oesophageal mucosal damage, which can progress to ulceration and stricture formation, although only about 8% will have moderate or severe oesophagitis.
Esophageal stricture
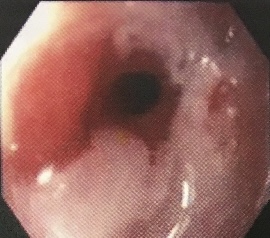
© డా. గన్నవరపు నరసింహమూర్తి, CC0, via Wikimedia Commons
డా. గన్నవరపు నరసింహమూర్తి, CC0, via Wikimedia Commons
Continue reading below
Causes of GORD (aetiology)1
Back to contentsFactors that predispose to reflux include:
Increased intra-abdominal pressure.
Smoking, alcohol, fat, chocolate, coffee.
Pregnancy.
Obesity.
Stress and anxiety.
Tight clothes.
Big meals.
Surgery in achalasia of the cardia.
Systemic sclerosis.
Drugs, including alpha-blockers, anticholinergics, benzodiazepines, beta-blockers, bisphosphonates, calcium-channel blockers, corticosteroids, nonsteroidal anti-inflammatory drugs, nitrates, theophyllines, and tricyclic antidepressants.
Family history.
Most of these predisposing factors increase intra-abdominal pressure and a fatty meal delays gastric emptying but the listed drugs and smoking relax the tone of the cardiac sphincter. NB: there is no proven relationship between Helicobacter pylori infection and GORD.
Bile is particularly caustic and reflux of duodenal contents is more troublesome than reflux of gastric contents alone. There is little correlation between severity of symptoms and findings on endoscopy.
Symptoms of GORD (presentation)1
Back to contentsHeartburn is a burning feeling, rising from the stomach or lower chest up towards the neck, that is related to meals, lying down, stooping and straining. It is relieved by antacids.
Retrosternal discomfort, acid brash - regurgitation of acid or bile.
Water brash - this is excessive salivation.
Odynophagia (pain on swallowing) may be due to severe oesophagitis or stricture.
Atypical symptoms
These include chest pain, epigastric pain and bloating.
Non-cardiac chest pain caused by GORD has been found in up to 50% of patients with chest pain and normal coronary angiography. Usually there is no relationship to exercise and this helps to differentiate most cases of reflux-induced chest pain from true angina.
Respiratory symptoms include chronic hoarseness (the Cherry-Donner syndrome), chronic cough, and asthmatic symptoms like wheezing and shortness of breath. Episodic or chronic aspiration can cause pneumonia, lung abscess and interstitial pulmonary fibrosis. In 6-10% of patients with chronic cough, GORD is the underlying cause.
Continue reading below
Diagnosing GORD (investigations)1
Back to contentsEndoscopy is the investigation of choice.
Perform FBC to exclude significant anaemia.
Barium swallow may show hiatus hernia (fluid level on CXR does not prove oesophagitis).
Oesophageal manometry may help exclude oesophageal motility disorders such as achalasia and severe oesophageal hypomotility. Should also be undertaken if anti-reflux surgery is being considered.
Oesophageal pH monitoring to assess if symptoms coincide with acid in the oesophagus. This can be done with:
Naso-oesophageal pH catheter (24-hour study).
Wireless pH capsule (Bravo®).4
Oesophageal impedance and pH via nasal catheter (can give quantitative information about the amount of fluid refluxed).
Endoscopic grading of oesophagitis
Important information |
|---|
The Savary-Miller grading system is commonly used:5 Grade 1: single or multiple erosions on a single fold. Erosions may be exudative or erythematous. Grade 2: multiple erosions affecting multiple folds. Erosions may be confluent. Grade 3: multiple circumferential erosions. Grade 4: ulcer, stenosis or oesophageal shortening. Grade 5: Barrett's epithelium. Columnar metaplasia in the form of circular or non-circular (islands or tongues) extensions. Grade A: one or more mucosal breaks no longer than 5 mm, none of which extends between the tops of the mucosal folds. Grade B: one or more mucosal breaks more than 5 mm long, none of which extends between the tops of two mucosal folds. Grade C: mucosal breaks that extend between the tops of two or more mucosal folds but which involve <75% of the mucosal circumference. Grade D: mucosal breaks which involve ≥75% of the mucosal circumference. |
Differential diagnosis
Back to contentsOesophagitis from swallowed corrosives or drugs like NSAIDs.
Infection (especially in the immunocompromised): cytomegalovirus, herpes, candidiasis.
Gastrointestinal (GI) cancers.
Non-ulcer dyspepsia.
Management of GORD7
Back to contentsThe National Institute for Health and Care Excellence (NICE) in the UK has published guidelines on the management of dyspepsia (including reflux symptoms) which impact on clinical practice.
NICE guidelines for urgent referral for suspected upper GI cancer8
Dysphagia - food sticking on swallowing, at any age.
Dyspepsia at any age combined with one or more of the following 'alarm' symptoms:
Weight loss
Proven anaemia
Vomiting
Dyspepsia in a patient aged 55 years or more with at least one of the following 'high-risk' features:
Onset of dyspepsia <1 year previously.
Continuous symptoms since onset.
Dyspepsia combined with at least one of the following known 'risk factors':
Family history of upper GI cancer in more than two first-degree relatives.
Barrett's oesophagitis.
Pernicious anaemia.
Peptic ulcer surgery over 20 years previously.
Known dysplasia, atrophic gastritis, intestinal metaplasia.
Jaundice.
Upper abdominal mass.
One study of the predictive value of alarm symptoms reported that upper GI bleeding, persistent vomiting and odynophagia were specific for significant endoscopic findings. The pooled sensitivity, specificity, positive predictive value, and negative predictive value of the alarm features were 65%, 49%, 71% and 41% respectively.9
Routine endoscopic investigation of dyspepsia is not necessary for patients (of any age) without alarm symptoms.
Lifestyle
The evidence base supporting the effectiveness of giving people with dyspepsia lifestyle advice is small but should nevertheless be given.10 The following are recommended:
Reduce weight.
Stop smoking.
Reduce alcohol intake.
Raise the head of the bed at night.
Take small, regular meals.
Avoid hot drinks, alcohol and eating during the three hours before going to bed.
Avoid drugs which affect oesophageal motility (nitrates, anticholinergics, tricyclic antidepressants) or damage the mucosa (NSAIDs, potassium salts, alendronate).
Pharmacological treatment
Patients with reflux symptoms but no alarm symptoms, should receive initial treatment with full-dose proton pump inhibitors (PPIs) for one month.
In cases of uninvestigated dyspepsia, eradication therapy for H. pylori can also be provided if infection is evident on serology or urea breath test. Where there is known GORD (ie post-gastroscopy), H. pylori eradication is not recommended.
If symptoms return after treatment, and long-term acid suppression is required, a step-down strategy to the lowest dose of PPI that provides effective relief of symptoms is more cost-effective than the step-up approach . Start acid suppression at a healing dose for one to two months. Then either step up a level if still symptomatic, or step down, once symptoms have improved, to the lowest level that provides effective symptom control. All patients should have a treatment plan and should be told if they can stop if symptom-free.
Referral for endoscopy
It may become appropriate to refer some patients with an inadequate response to therapy, or new emergent symptoms, to a specialist for a second opinion.
Review medications for possible causes of dyspepsia; for example, calcium antagonists, nitrates, theophyllines, bisphosphonates, steroids and NSAIDs. Patients undergoing endoscopy should be free from medication with either a PPI or an H2-receptor antagonist (H2RA) for a minimum of two weeks.
Consider the possibility of cardiac or biliary disease as part of the differential diagnosis.
Post-endoscopy
If endoscopy is carried out and oesophagitis is present, a healing dose of PPI should be prescribed for two months.
In such patients, symptoms usually relapse when treatment is withdrawn, and maintenance PPI therapy is usually required.
PPIs are more effective than H2RAs in relieving heartburn in patients with GORD who are treated empirically and in those with endoscopy-negative reflux disease, although the degree of benefit is greater for those treated empirically.11 The long-term safety of PPIs however has been subject to debate. Emerging evidence from multiple observational studies suggests long-term use of PPIs is associated with a higher risk of gastric cancer development. This does not mean that PPIs should be universally banned from long-term use; rather that they should be tailored to the individual's risk-benefit profile. The risk is likely limited to individuals with current or past history of H. pylori infection, particularly those with underlying pre-cancerous gastric lesions. Patients in genuine need - eg, those with Barrett’s oesophagus or high risk of upper GI bleeding - should not be denied the benefits of long-term PPI therapy. Further prospective research is needed.12
Surgery
Most patients with reflux symptoms are treated with lifestyle interventions and medication. Laparascopic fundoplication has been advocated in the past but a 2015 Cochrane review concluded there was considerable uncertainty in the balance of benefits versus harms compared to long-term medical treatment with PPIs.13 Since this review, the association between long-term PPI therapy and gastric cancer has come to the fore, and there is clearly a need for prospective studies to compare the risk-benefit ratio of both therapeutic approaches.
Laparoscopic insertion of a magnetic bead band:14
With the patient under general anaesthesia, an implant is placed so that it encircles the distal oesophagus at the gastro-oesophageal junction.
The implant is then secured in place. The implant consists of a ring of interlinked titanium beads, each with a weak magnetic force which holds the beads together to keep the distal oesophagus closed.
When the patient swallows, the magnetic force is overcome, allowing the ring to open. After swallowing, magnetic attraction brings the beads together and the distal oesophagus is again closed.
MRI is contra-indicated after this procedure.
NICE currently recommends that the evidence on the safety and efficacy of laparoscopic insertion of a magnetic ring for GORD is adequate to support using this procedure provided that standard arrangements are in place for clinical governance, consent and audit.15
Management problems
Symptoms persist in a minority of patients despite PPI therapy and this group remains a challenge to treat.
Some evidence suggests that once patients develop the disease, severity is determined early and patients seem to continue with that phenotype long-term.16 Therapeutic options include:
Doubling the dose of PPI therapy.
Adding an H2RA at bedtime.
Extending the length of treatment.
Surgery (see section above).
Specific groups should be given continuous, rather than intermittent, therapy:
Patients with a documented NSAID-induced ulcer, who must unavoidably continue with NSAIDs (eg, severe rheumatoid arthritis), should remain on maintenance doses of PPIs.
Patients with severe reflux oesophagitis should remain on maintenance doses of PPI to prevent its recurrence.
Patients with complicated reflux disease (stricture, ulcer, haemorrhage) should be left on 'full-dose' PPI.
The cheapest effective PPI should be used.
NB: sudden or progressive worsening of symptoms in a patient aged over 55 years, or the development of dysphagia, anaemia, persistent vomiting or weight loss at any age, merits urgent referral for endoscopy (two-week rule - as per local guidelines).
Prokinetic agents are no longer recommended. However, several new-generation prokinetics such as acotiamide are emerging. Acotiamide has received approval for use in Japan and is currently being evaluated in Europe.17
Laryngopharyngeal reflux2
Medical therapies include proton pump inhibitors, H2 receptor antagonists, alginates, and baclofen.
Other non-invasive treatment options include lifestyle advice |(as for GORD) and an external upper oesophageal sphincter compression device. Endoscopic and surgical options include anti-reflux surgery, magnetic sphincter augmentation, and transoral incisionless fundoplication.
Complications of GORD1
Back to contentsOesophagitis/ulcer.
Oesophageal haemorrhage.
Anaemia due to chronic blood loss.
Aspiration pneumonia.
Oral problems, eg, dental erosions, gingivitis, and halitosis.
Barrett's oesophagus: 10–15% of people with GORD symptoms will develop Barrett's oesophagus, of these 1–10% will develop oesophageal adenocarcinoma over the following 10–20 years.
Prognosis1
Back to contentsAnnual risk of recurrence of untreated GORD symptoms is 50%, and the lifetime recurrence risk is 80%.
60–80% of people with successfully treated GORD symptoms will relapse within 1 year if not given maintenance therapy.
Relapse is more likely in people with severe oesophagitis.
About 10% of people with endoscopy-negative reflux disease will develop severe oesophagitis over time.
Further reading and references
- Katz PO, Dunbar KB, Schnoll-Sussman FH, et al; ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol. 2022 Jan 1;117(1):27-56. doi: 10.14309/ajg.0000000000001538.
- Gyawali CP, Yadlapati R, Fass R, et al; Updates to the modern diagnosis of GERD: Lyon consensus 2.0. Gut. 2024 Jan 5;73(2):361-371. doi: 10.1136/gutjnl-2023-330616.
- Endoluminal gastroplication for gastro-oesophageal reflux disease; NICE Interventional Procedure Guidance, July 2011
- Endoscopic radiofrequency ablation for gastro-oesophageal reflux disease; NICE Interventional Procedure Guidance, August 2013
- Dyspepsia - proven GORD; NICE CKS, July 2023 (UK access only)
- Krause AJ, Walsh EH, Weissbrod PA, et al; An update on current treatment strategies for laryngopharyngeal reflux symptoms. Ann N Y Acad Sci. 2022 Apr;1510(1):5-17. doi: 10.1111/nyas.14728. Epub 2021 Dec 17.
- Wilson JA, Stocken DD, Watson GC, et al; Lansoprazole for persistent throat symptoms in secondary care: the TOPPITS RCT. Health Technol Assess. 2021 Jan;25(3):1-118. doi: 10.3310/hta25030.
- Iluyomade A, Olowoyeye A, Fadahunsi O, et al; Interference with daily activities and major adverse events during esophageal pH monitoring with bravo wireless capsule versus conventional intranasal catheter: a systematic review of randomized controlled trials. Dis Esophagus. 2017 Feb 1;30(3):1-9. doi: 10.1111/dote.12464.
- Genta RM, Spechler SJ, Kielhorn AF; The Los Angeles and Savary-Miller systems for grading esophagitis: utilization and correlation with histology. Dis Esophagus. 2011 Jan;24(1):10-7. doi: 10.1111/j.1442-2050.2010.01092.x.
- Liu L, Li S, Zhu K, et al; Relationship between esophageal motility and severity of gastroesophageal reflux disease according to the Los Angeles classification. Medicine (Baltimore). 2019 May;98(19):e15543. doi: 10.1097/MD.0000000000015543.
- Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management; NICE Clinical Guideline (Sept 2014 - last updated October 2019)
- Suspected cancer: recognition and referral; NICE guideline (2015 - last updated January 2026)
- Odeghe EA, Adeniyi OF, Oyeleke GK, et al; Use of alarm features in predicting significant endoscopic findings in Nigerian patients with dyspepsia. Pan Afr Med J. 2019 Oct 2;34:66. doi: 10.11604/pamj.2019.34.66.18848. eCollection 2019.
- Feinle-Bisset C, Azpiroz F; Dietary and lifestyle factors in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013 Mar;10(3):150-7. doi: 10.1038/nrgastro.2012.246. Epub 2013 Jan 8.
- Sigterman KE, van Pinxteren B, Bonis PA, et al; Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013 May 31;(5):CD002095. doi: 10.1002/14651858.CD002095.pub5.
- Cheung KS, Leung WK; Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol. 2019 Mar 11;12:1756284819834511. doi: 10.1177/1756284819834511. eCollection 2019.
- Garg SK, Gurusamy KS; Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev. 2015 Nov 5;(11):CD003243. doi: 10.1002/14651858.CD003243.pub3.
- Nencioni M, Asti E, Saino G, et al; Magnetic oesophageal sphincter for the treatment of gastro-oesophageal reflux disease: results of a prospective clinical trial. Chir Ital. 2009 Mar-Apr;61(2):187-92.
- Laparoscopic insertion of a magnetic ring for gastro-oesophageal reflux disease; Interventional procedures guidance, January 2023
- Moayyedi P, Talley NJ; Gastro-oesophageal reflux disease. Lancet. 2006 Jun 24;367(9528):2086.
- Nakamura K, Tomita T, Oshima T, et al; A double-blind placebo controlled study of acotiamide hydrochloride for efficacy on gastrointestinal motility of patients with functional dyspepsia. J Gastroenterol. 2017 May;52(5):602-610. doi: 10.1007/s00535-016-1260-7. Epub 2016 Sep 17.
Continue reading below
Article history
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 15 Aug 2027
16 Aug 2024 | Latest version

Ask, share, connect.
Browse discussions, ask questions, and share experiences across hundreds of health topics.

Feeling unwell?
Assess your symptoms online for free