Intrauterine contraceptives (IUD and LNG-IUD) - management
Peer reviewed by Dr Doug McKechnie, MRCGPLast updated by Dr Toni Hazell, MRCGPLast updated 30 Jan 2024
Meets Patient’s editorial guidelines
- DownloadDownload
- Share
- Language
- Discussion
Medical Professionals
Professional Reference articles are designed for health professionals to use. They are written by UK doctors and based on research evidence, UK and European Guidelines. You may find the Intrauterine contraceptive device article more useful, or one of our other health articles.
In this article:
Continue reading below
What is an intrauterine contraceptive device?
An intrauterine contraceptive device (IUD) is a small device made of plastic that is placed into the uterus as an effective method of contraception. There are two types - the copper IUD (Cu-IUD) and the levonorgestrel IUD (LNG-IUD).
This article covers the theory related to the practicalities of inserting these devices. It is not a substitute for practical training: insertion of intrauterine devices should only be undertaken by an appropriately trained family planning professional who maintains competence by fitting at least one IUD/LNG-IUD per month and keeps up to date.
In the Americas, IUDs are used by well below 10% of women of reproductive age. In the UK their use is between 11.3 and 12.1%.1
Intrauterine contraceptive types
Back to contentsThere are two types of intrauterine contraceptives available for use in the UK:
Copper-releasing devices - multiple brands of these are available.
Levonorgestrel-releasing intrauterine system (LNG-IUD) - there are 5 brands available, Mirena®, Benilexa®, Levosert®, Kyleena® and Jaydess®.
Indications, contra-indications and side-effects of each device are listed in the respective separate Intrauterine Contraceptive Device and Levonorgestrel intrauterine device (LNG-IUD) articles.
Continue reading below
Choice of device
Back to contentsAfter counselling, most women can choose between a Cu-IUD and a LNG-IUD.
If a woman chooses a Cu-IUD then the device with the longest duration of use and lowest failure rate should be used first-line.
If the uterus sounds to 6.5 cm or less then a device with a shorter stem or a frameless device may be used.
There is no evidence suggesting that a different choice should be made in nulliparous women.
Timing of IUD/LNG-IUD insertion
Back to contentsBoth types of IUD may be inserted at any time in the menstrual cycle, as long as pregnancy can reasonably be excluded (see 'Excluding pregnancy', below). Documenting a negative pregnancy test may be sensible, if possible.
If the LNG-IUD is not inserted between day 1 and day 5 of the menstrual cycle, condoms are advised for the first seven days of use.2
Insertion towards the end of the menstrual period, or just after it, may be more comfortable for the patient.
Excluding pregnancy
Pregnancy may be reasonably excluded if the woman meets any of the following criteria: 3 2
She has not had intercourse since last the normal menses or since childbirth, abortion, miscarriage, ectopic pregnancy or uterine evacuation for gestational trophoblastic disease (GTD).
She has been correctly and consistently using a reliable method of contraception, including barrier methods if they have been correctly used for every episode of intercourse.
She is in day 1-5 of a normal menstrual cycle.
She is in day 1-5 following termination, ectopic, uterine evacuation for GTD or miscarriage.
She is fully breastfeeding, amenorrhoeic and within six months of delivery. However, even if these criteria are all met, the failure rate of breastfeeding as a contraceptive method has a failure rate of 2% and so many women will also want to use an additional method.
She has not had intercourse for ≥ 21 days and has a negative pregnancy test.
Postpartum insertion
UK medical eligibility criteria state that risks generally outweigh benefits if postpartum insertion occurs between 48 hours and four weeks.4 This reflects an increased rate of uterine perforation due to the softness of the postpartum uterus. Expulsion of the device is more common for insertions after 48 hours post-delivery.
Beyond four weeks postpartum, benefits outweigh risks, even if the woman is breastfeeding (there is no increased copper level in breast milk) and in post-caesarean section mothers.
Insertion during or after termination of pregnancy2
Insertion during surgical termination of pregnancy is safe and practical. It can often be a convenient time to perform the procedure and avoids discomfort. Expulsion rates are marginally increased.
Emergency contraception5
A Cu-IUD (or advice on how to obtain one) should be offered to all women attending for emergency contraception (EC) even if presenting within 72 hours of UPSI, as the failure rate is significantly lower than for oral EC.
IUDs with banded copper on the arms and containing at least 380 mm2 of copper have the lowest failure rates for EC and should be the first-line choice.
Ideally, an emergency IUD should be fitted at first presentation, but insertion can be offered later, at the woman's convenience or if this is necessary for service provision. In this case, oral EC should be given in the interim.
A Cu-IUD can be inserted up to five days after the first episode of UPSI. If the timing of ovulation can be reliably estimated, insertion can be beyond five days of UPSI, as long as it does not occur beyond five days after the estimated date of ovulation.
The LNG-IUD is not suitable for EC.
Continue reading below
Pre-insertion counselling
Back to contentsBefore the device is fitted, patients should be offered advice on the following:
Mode of action: a Cu-IUD primarily prevents fertilisation through the toxic effect of copper on the sperm and the egg; copper in the cervical mucus also reduces the passage of sperm into the uterus, and there is an inflammatory endometrial response which can prevent implantation. The LNG-IUD also reduces the passage of sperm into the uterus, through the effects of the progestogen, and may reduce implantation due to effects on the endometrium. In some women, ovulation will be inhibited.
Failure rates (0.8% for the Cu-IUD and 0.2% for the LNG-IUD for typical use in the first year). See also the separate Intrauterine Contraceptive Device article.3
Perforation risk (0-2 per 1,000 insertions).2
Expulsion risk (1 in 20, most commonly in the first three months after fitting).2
No delay in return to fertility post-removal, although there may be a delay in return of regular menses after a LNG-IUD is removed.
Increased risk of pelvic infection in the three weeks after fitting, but absolute risk is still low (<1%).2
Irregular bleeding and sometimes pain are common for the first 3-6 months of IUD use. These can be managed, and if they are discussed at counselling then women are more likely to anticipate them and seek help, rather than removal, if they occur. Irregular bleeding and spotting often continue for the first six months of LNG-IUD use, although oligomenorrhoea or amenorrhoea are usual within a year.
Hormonal side-effects from the LNG-IUD are uncommon and rarely a reason to discontinue
The insertion procedure may be uncomfortable; oral analgesia prior to insertion may be helpful
STI screening: a clinical history is required prior to insertion, to assess the individual sexual health risk for each woman.
Examination and testing for STIs - Chlamydia trachomatis and Neisseria gonorrhoeae - may then be offered and performed, if appropriate.
Testing for any STIs should be offered in women who request it.
Insertion6
Back to contentsPreparation
Obtain informed consent. Oral consent is acceptable.
Offer a chaperone.
An appropriately trained assistant should be present (to monitor the patient and assist in an emergency) - this is a 'should' rather than a 'must' statement - if an assistant is not available then the fitter must be certain that they can rapidly summon help without leaving the patient.
Basic risk assessment includes gathering information about previous intrauterine procedures. Patients who have had previous adverse events during insertion may be more likely to have them again.
The routine documentation of pulse rate and blood pressure is not required.
The need for pain relief during insertion should be discussed with the patient.
Routine antibiotic prophylaxis is not recommended pre-insertion. However, for women at increased risk of STIs, in whom testing has not been completed, prophylactic antibiotics are advised.
Antibiotic prophylaxis is no longer recommended for women at risk of endocarditis - eg, those who have had previous endocarditis or who have a prosthetic heart valve or valvular heart disease. This does not mean that there is no risk, and the woman's cardiologist should be consulted if there are any concerns.
For women with symptomatic pelvic infection, treatment should be given prior to insertion, which should be postponed until symptoms resolve.
Cervical cleansing should be performed with antiseptic solution.
A 'no-touch' sterile technique should be adopted.
A pelvic examination should be performed prior to inserting the device, to assess the size, shape, position and mobility of the uterus.
Assessment of uterine length should be undertaken using a uterine sound.
A tenaculum is used to stabilise the cervix during insertion and reduce the risk of perforation.
Some operators offer cervical anaesthesia for the procedure.
Insertion may be offered under general anaesthesia to patients who cannot tolerate the procedure.
Documentation should be made in the case notes of pre- and post-insertion counselling, the procedure, the type of device inserted, and any adverse events.
Procedure: copper-releasing devices
Most IUDs will have a leaflet within the box, describing insertion, with diagrams - mechanism varies by brand and so will not be further discussed here.
IUD in place
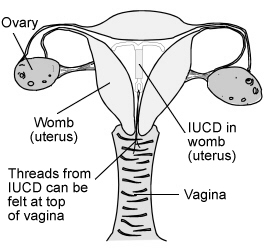
Procedure: LNG-IUD
The LNG-IUD has a leaflet within the box, outlining the process. Mechanism varies by brand and so will not be further discussed here.
Problems associated with insertion
Back to contentsInsertion failure
Pain during insertion is associated with a higher failure rate.
Other factors relate to the experience of the doctor.
Difficulties include problems passing the sound or IUD through the cervix and immediate expulsion.
Syncope
Syncope may be experienced at insertion secondary to vagal stimulation from the cervix.
Bradycardia is more common in nulliparous women.
Suspected perforation at insertion
The rate of perforation in experienced operators is low 1-2 per 1,000 insertions.2
The risk of perforation, whilst still small, is significantly increased in lactating women.
Symptoms are most commonly of abdominal pain (which is usually mild) associated with lost threads and abnormal bleeding.
Most perforations are not recognised at the time of insertion and are discovered when there is a shortening or disappearance of strings, or a pregnancy.
Some patients are asymptomatic and the situation may be discovered only because the threads are lost.
More severe pain can occur, and severe complications have been described including damage to viscera and peritonitis.
The device cannot be considered effective if perforation has occurred, so pregnancy is a further complication.
Perforation rates are greatest during the postpartum period.
If perforation is suspected at the time of insertion, stop the procedure and check vital signs. If these are altered, monitor until stable or seek help if the patient is acutely unwell. Ultrasound or plain abdominal film to locate the device should be arranged as soon as possible (copper devices have flexible side arms containing barium sulfate which is visible on X-ray).
Copper ions are inflammatory, and there are reports of IUDs eroding into the bladder or gastrointestinal tract. It is therefore recommended that the IUD be retrieved as soon as possible.
Vasovagal collapse/cervical shock
Abandon the procedure, lower the head and/or raise the legs.
The IUD may need to be removed.
An assistant should monitor vital signs.
Ensure a clear airway.
Oxygen and suction as required.
Consider use of atropine 0.6 mg/ml IV for persistent bradycardia.
Consider use of adrenaline (epinephrine) 1:1000 IM (1 mg/nl) for management of anaphylaxis.
Automated external defibrillator (AED) should be available.
Arrange ambulance transfer if there is no swift improvement.
Problems occurring after insertion
Back to contentsCramping
This is common for the first 24-48 hours and does not usually persist beyond this.
Expulsion
This can occur immediately. It is more likely where the procedure is less well tolerated and in nulliparous women, but is also related to the skill of the operator.
Overall it occurs in 1 in 20 women, most commonly in the first three months during menstruation.2 The patient may be unaware that expulsion has taken place.
Expulsion is more common with copper devices, younger patients and nulliparous patients.
Expulsion is most likely to occur in the first 72 hours post-insertion, and may in some cases relate to a widened cervical os.
Infection
The risk of pelvic inflammatory disease (PID) in association with an IUD is related to the insertion - it is higher than baseline for the first 3 weeks, but the absolute risk remains < 1%.
Lost threads 3
If threads are not visible, or if they are but the stem of the device is palpable, the woman should be advised to use condoms or abstain from intercourse until the site of the device (if present) can be determined.
Perform a speculum examination to ensure the device is not in the posterior fornix.
Determine whether the woman is already pregnant - if she has had sex in the last 21 days then a pregnancy test may need to be repeated 21 days after the last intercourse in which an alternative method of contraception was not used.
With consent, explore the lower part of the endocervical canal with narrow artery forceps: threads which have been drawn a little way up are usually found by this method.
An experienced operator may, after appropriate analgesia (eg, mefenamic acid 500 mg) explore the uterine cavity with a retriever hook.
Hormonal emergency contraception may be indicated.
Ultrasound should be arranged to locate the device.
If ultrasound does not locate the device and there is no definite history of expulsion then abdominal X-ray should be performed to look for an extrauterine device.
Expulsion should not otherwise be assumed.
Hysteroscopy can be helpful if ultrasound is equivocal.
Surgical retrieval of an extrauterine device is advised.
Management of ongoing problems
See the separate Intrauterine Contraceptive Device article, which discusses management of problems which may be associated with IUD use, and the separate Intrauterine System article which discusses management of problems which may be associated with LNG-IUD use.
Post-insertion advice
Back to contentsInstruct the patient on how to feel the threads, and advise her to seek medical advice if she is unable to feel them. If the woman can feel the threads, and has no symptoms which concern her, there is no need for a routine check at six-weeks, which was previously done as a routine.
Check the patient is feeling well enough to leave.
Make sure that the woman is aware of when the IUD will need to be changed- this may vary depending on the indication. For example, some brands of LNG-IUD can be used for up to 8 years for contraception,7 but only for 5 years if used as the progestogenic component of HRT. Where there are 2 concurrent indications for use, the device should be replaced at the shorter time interval.
Documentation
Back to contentsIt is advised that the following documentation be kept for IUD insertion:
Medical history and clinical assessment
Age.
Menstrual history (including date of last menstrual period).
Previous contraception used (including difficulty in IUD insertion).
Obstetric history (including ectopic pregnancy).
Past medical history (relevant cardiovascular disease, past gynaecological history/cervical surgery, including treatment to the cervix, history of STIs and PID, relevant medical history and conditions, allergies).
Coital history.
Sexual history to identify risk of STIs.
Information advice and counselling
Contraceptive choices discussed.
Risks/benefits/uncertainties discussed.
Mode of action and efficacy of IUD, choice of device and duration of use.
Effects on bleeding pattern.
Risk of spontaneous expulsion and perforation.
Risk of post-insertion pelvic infection.
Explanation of insertion procedure, consent obtained, leaflets given, including manufacturer's patient information.
Thread check and teaching.
Details of insertion procedure
Name of assistant.
Any tests undertaken.
Bimanual examination and speculum findings.
Analgesia/local anaesthesia if used.
Allis forceps application, uterine sounding/uterocervical length, use of 'no-touch' technique, problems encountered, if any, and actions taken.
Type of device inserted/removed and date for removal.
Post-insertion follow-up advice
Other treatment if any (eg, antibiotics; special instructions if any, such as postcoital IUD).
Follow-up if there are any problems or threads cannot be felt.
Details of removal.
Reason for removal.
Coital history (since last menstrual period) to identify risk of pregnancy post-removal.
Alternative contraception method advised/provided, if any.
Technique of removal used; problems encountered, if any, and actions taken.
Dr Mary Lowth is an author or the original author of this leaflet.
Dr Hazell was the eLearning fellow on the current RCGP eLearning course on contraception, which was developed in partnership with Public Health England and the Faculty of Sexual and Reproductive Healthcare. She is the chair of the RCGP women's health library, which involves liaison with the FSRH. She has written and spoken on contraception on multiple occasions - some of these engagements were funded by pharmaceutical companies which may make copper intrauterine devices.
Further reading and references
- Firman N, Palmer MJ, Timaeus IM, et al; Contraceptive method use among women and its association with age, relationship status and duration: findings from the third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3). BMJ Sex Reprod Health. 2018 May 25. pii: bmjsrh-2017-200037. doi: 10.1136/bmjsrh-2017-200037.
- Intrauterine Contraception; Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit (March 2023 - last updated July 2023)
- Contraception - IUC; NICE CKS, January 2024 (UK access only)
- UK Medical Eligibility Criteria Summary Table for intrauterine and hormonal contraception; Faculty of Sexual and Reproductive Healthcare, 2016 - amended September 2019
- CEU Clinical Guidance: Emergency Contraception; Faculty of Sexual and Reproductive Healthcare (March 2017 - updated July 2023)
- IUD/IUS; Lothian Sexual Health
- FSRH CEU Statement: Mirena® 52mg LNG-IUD extension of licence for contraception to 8 years; FSRH, January 2024
Continue reading below
Article history
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 28 Jan 2029
30 Jan 2024 | Latest version

Ask, share, connect.
Browse discussions, ask questions, and share experiences across hundreds of health topics.

Feeling unwell?
Assess your symptoms online for free